Abstract
Background
Pancreatic neuroendocrine neoplasms (PanNENs) are a heterogeneous group of tumors. Although the prognosis of resected PanNENs is generally considered to be good, a relatively high recurrence rate has been reported. Given the scarcity of large-scale reports about PanNEN recurrence due to their rarity, we aimed to identify the predictors for recurrence in patients with resected PanNENs to improve prognosis.
Methods
We established a multicenter database of 573 patients with PanNENs, who underwent resection between January 1987 and July 2020 at 22 Japanese centers, mainly in the Kyushu region. We evaluated the clinical characteristics of 371 patients with localized non-functioning pancreatic neuroendocrine tumors (G1/G2). We also constructed a machine learning-based prediction model to analyze the important features to determine recurrence.
Results
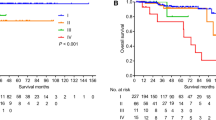
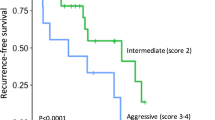
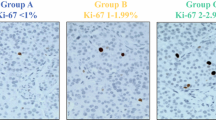
Fifty-two patients experienced recurrence (14.0%) during the follow-up period, with the median time of recurrence being 33.7 months. The random survival forest (RSF) model showed better predictive performance than the Cox proportional hazards regression model in terms of the Harrell’s C-index (0.841 vs. 0.820). The Ki-67 index, residual tumor, WHO grade, tumor size, and lymph node metastasis were the top five predictors in the RSF model; tumor size above 20 mm was the watershed with increased recurrence probability, whereas the 5-year disease-free survival rate decreased linearly as the Ki-67 index increased.
Conclusions
Our study revealed the characteristics of resected PanNENs in real-world clinical practice. Machine learning techniques can be powerful analytical tools that provide new insights into the relationship between the Ki-67 index or tumor size and recurrence.





Similar content being viewed by others
Conflict of interest The authors declare that they have no conflict of interest.References
Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Masui T, Ito T, Komoto I, et al. Recent epidemiology of patients with gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NEN) in Japan: a population-based study. BMC Cancer. 2020;20:1104.
Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64.
Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42.
Gill A, Klimstra D, Lam A, et al. WHO classification of tumours: Digestive system tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2019.
Fujimori N, Miki M, Lee L, et al. Natural history and clinical outcomes of pancreatic neuroendocrine neoplasms based on the WHO 2017 classification; a single-center experience of 30 years. Pancreatology. 2020;20:709–15.
Ito T, Masui T, Komoto I, et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol. 2021;56:1033–44.
Miki M, Oono T, Fujimori N, et al. CLEC3A, MMP7, and LCN2 as novel markers for predicting recurrence in resected G1 and G2 pancreatic neuroendocrine tumors. Cancer Med. 2019;8:3748–60.
Gao H, Liu L, Wang W, et al. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett. 2018;412:188–93.
Ye L, Ye H, Zhou Q, et al. A retrospective cohort study of pancreatic neuroendocrine tumors at single institution over 15 years: New proposal for low- and high-grade groups, validation of a nomogram for prognosis, and novel follow-up strategy for liver metastases. Int J Surg. 2016;29:108–17.
Landoni L, Marchegiani G, Pollini T, et al. The evolution of surgical strategies for pancreatic neuroendocrine tumors (Pan-NENs): time-trend and outcome analysis from 587 consecutive resections at a high-volume institution. Ann Surg. 2019;269:725–32.
Yamamoto Y, Okamura Y, Uemura S, et al. Vascularity and tumor size are significant predictors for recurrence after resection of a pancreatic neuroendocrine tumor. Ann Surg Oncol. 2017;24:2363–70.
Hashim YM, Trinkaus KM, Linehan DC, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg. 2014;259:197–203.
Li Y, Fan G, Yu F, et al. Meta-analysis of prognostic factors for recurrence of resected well-differentiated pancreatic neuroendocrine tumors. Neuroendocrinology. 2021;111:1231–7.
Tsutsumi K, Ohtsuka T, Fujino M, et al. Analysis of risk factors for recurrence after curative resection of well-differentiated pancreatic neuroendocrine tumors based on the new grading classification. J Hepatobiliary Pancreat Sci. 2014;21:418–25.
Kaneko H, Umakoshi H, Ogata M, et al. Machine learning based models for prediction of subtype diagnosis of primary aldosteronism using blood test. Sci Rep. 2021;11:9140.
Kaneko H, Umakoshi H, Ogata M, et al. Machine learning-based models for predicting clinical outcomes after surgery in unilateral primary aldosteronism. Sci Rep. 2022;12:5781.
Zhou RQ, Ji HC, Liu Q, et al. Leveraging machine learning techniques for predicting pancreatic neuroendocrine tumor grades using biochemical and tumor markers. World J Clin Cases. 2019;7:1611–22.
Luo Y, Chen X, Chen J, et al. Preoperative prediction of pancreatic neuroendocrine neoplasms grading based on enhanced computed tomography imaging: validation of deep learning with a convolutional neural network. Neuroendocrinology. 2020;110:338–50.
Song Y, Gao S, Tan W, et al. Multiple machine learnings revealed similar predictive accuracy for prognosis of PNETs from the surveillance, epidemiology, and end result database. J Cancer. 2018;9:3971–8.
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95: 103208.
Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Murakami M, Fujimori N, Matsumoto K, et al. A clinical analysis on functioning pancreatic neuroendocrine tumors (focusing on VIPomas): a single-center experience. Endocr J. 2022. https://doi.org/10.1507/endocrj.EJ22-0111.
van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6.
Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17:53.
Kronek LP, Reddy A. Logical analysis of survival data: Prognostic survival models by detecting high-degree interactions in right-censored data. Bioinformatics. 2008;24:i248–53.
Chen J, Yang Y, Liu Y, et al. Prognosis analysis of patients with pancreatic neuroendocrine tumors after surgical resection and the application of enucleation. World J Surg Oncol. 2021;19:11.
Zheng-Pywell R, Fang A, AlKashash A, et al. Prognostic impact of tumor size on pancreatic neuroendocrine tumor recurrence may have racial variance. Pancreas. 2021;50:347–52.
Zhang XF, Wu Z, Cloyd J, et al. Margin status and long-term prognosis of primary pancreatic neuroendocrine tumor after curative resection: results from the US neuroendocrine tumor study group. Surgery. 2019;165:548–56.
Kwon W, Jang JY, Song KB, et al. Risk factors for recurrence in pancreatic neuroendocrine tumor and size as a surrogate in determining the treatment strategy: a Korean nationwide study. Neuroendocrinology. 2021;111:794–804.
Dong DH, Zhang XF, Lopez-Aguiar AG, et al. Resection of pancreatic neuroendocrine tumors: defining patterns and time course of recurrence. HPB (Oxford). 2020;22:215–23.
Marchegiani G, Landoni L, Andrianello S, et al. Patterns of recurrence after resection for pancreatic neuroendocrine tumors: who, when, and where? Neuroendocrinology. 2019;108:161–71.
Jilesen AP, van Eijck CH, in’t Hof KH, et al. Postoperative complications, in-hospital mortality and 5-year survival after surgical resection for patients with a pancreatic neuroendocrine tumor: a systematic review. World J Surg. 2016;40:729–48.
Sadot E, Reidy-Lagunes DL, Tang LH, et al. Observation versus resection for small asymptomatic pancreatic neuroendocrine tumors: a matched case-control study. Ann Surg Oncol. 2016;23:1361–70.
Breiman L. Random forests. Mach Learn. 2001;45:5–32.
Ishwaran H, Kogalur UB, Blackstone EH, et al. Random survival forests. Ann Appl Stat. 2008. https://doi.org/10.1214/08-AOAS169.
Sakin A, Tambas M, Secmeler S, et al. Factors affecting survival in neuroendocrine tumors: a 15-year single center experience. Asian Pac J Cancer Prev. 2018;19:3597–603.
Lee L, Igarashi H, Fujimori N, et al. Long-term outcomes and prognostic factors in 78 Japanese patients with advanced pancreatic neuroendocrine neoplasms: a single-center retrospective study. Jpn J Clin Oncol. 2015;45:1131–8.
Acknowledgements
We thank Hiroki Kaneko, Akihisa Ohno, Kazuhide Matsumoto, Katsuhito Teramatsu, and Ayumu Takeno (Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan), Makoto Hinokuchi, Issei Kojima, and Shiroh Tanoue (Digestive and Lifestyle Diseases, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan), Takao Ohtsuka (Department of Digestive Surgery, Breast and Thyroid Surgery, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan), Motohiro Yoshinari, and Yukiko Uramoto (Department of Gastroenterology and Hepatology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan), Kazunari Murakami, Teiziro Hirashita, and Masafumi Inomata (Department of Gastroenterology, Faculty of Medicine, Oita University, Oita, Japan), Yoshihiro Hamada, Naoaki Tsuchiya, Takehiko Koga, Takanori Kitaguchi, Takahide Sasaki, Ryo Nakashima, Fuminori Ishii, and Masatoshi Kajiwara (Department of Gastroenterology and Medicine, Faculty of Medicine, Fukuoka University, Fukuoka, Japan), Yasuhisa Ando (Department of Gastroenterological Surgery, Faculty of Medicine, Kagawa University, Kita-gun, Japan), Yoshinobu Okabe, Hiroya Terabe, and Shingo Hirai (Division of Gastroenterology, Department of Medicine, Kurume University School of Medicine, Kurume, Japan), Yu Takamatsu (Neuroendocrine Tumor Centre, Fukuoka Sanno Hospital, Fukuoka, Japan), Masayuki Hijioka (Department of Gastroenterology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan), Yusuke Watanabe (Department of Surgery, Hamanomachi Hospital, Fukuoka, Japan), Toyoma Kaku (Department of Gastroenterology, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan), Yuichi Tachibana (Department of Internal Medicine, Saiseikai Fukuoka General Hospital, Fukuoka, Japan), and Ryuichiro Kimura (Department of Surgery, Miyazaki Prefectural Miyazaki Hospital, Miyazaki, Japan) for their contributions to the study management, and Masayuki Hirose (Center for Clinical and Translational Research at Kyushu University Hospital) for reviewing the statistical methods used in this study. We also thank Editage (http://www.editage.com) for editing the manuscript draft.
Funding
This study was supported in part by JSPS KAKENHI (Grant No. JP22K08079), and the Smoking Research Foundation (Grant No. GAKF800505).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, and/or data collection. Data analyses were performed and the first draft of the manuscript was written by MM and NF, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murakami, M., Fujimori, N., Nakata, K. et al. Machine learning-based model for prediction and feature analysis of recurrence in pancreatic neuroendocrine tumors G1/G2. J Gastroenterol 58, 586–597 (2023). https://doi.org/10.1007/s00535-023-01987-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-01987-8