Abstract
Background
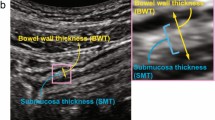
The development of feasible, reliable parameters and criteria for intestinal ultrasound (IUS) to estimate endoscopic remission of ulcerative colitis (UC) is a crucial clinical challenge. Such parameters must be simple, objective, and reproducible so that IUS can be widely used in daily practice. We developed a new parameter called the submucosa index (SMI), defined as a percentage of the submucosal thickness (SMT) in the total bowel wall thickness (BWT), and investigated its clinical potential.
Methods
The inclusion criteria were performance of both IUS and endoscopy (sigmoidoscopy or colonoscopy) for UC and a ≤ 15-day time interval between IUS and endoscopy. Loss of stratification was defined as inability to identify the submucosa even with a BWT of > 3 mm. The vascularity of the colon was assessed by the modified Limberg score (mLS) and evaluated as bowel wall flow (BWF) ( −) or ( +) using color Doppler mode. A Mayo endoscopic subscore (MES) of 0 or 1 was defined as endoscopic remission.
Results
Seventy-four colonic segments were analyzed. The SMI, mLS, and BWF could distinguish an MES of 1 versus 2 (p < 0.05, p < 0.01, and adjusted p < 0.001, respectively). The criteria using the BWT and SMI and using the BWT and BWF had the same estimating ability for endoscopic remission (sensitivity, 70.0%; specificity, 97.7%; positive predictive value, 95.5%; and negative predictive value, 82.7%).
Conclusion
The SMI is a practical, quantitative parameter based on the bowel wall structure and may be used to estimate endoscopic remission of UC.



Similar content being viewed by others
References
Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–83.
Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–35.
Goodsall TM, Nguyen TM, Parker CE, et al. Systematic review: gastrointestinal ultrasound scoring indices for inflammatory bowel disease. J Crohns Colitis. 2021;15:125–42.
Bots S, Nylund K, Lowenberg M, et al. Intestinal ultrasound to assess disease activity in ulcerative colitis: development of a novel UC-ultrasound index. J Crohns Colitis. 2021;15:1264–71.
Limberg B, Osswald B. Diagnosis and differential diagnosis of ulcerative colitis and Crohn’s disease by hydrocolonic sonography. Am J Gastroenterol. 1994;89:1051–7.
Stewart SF. Effects of transducer, velocity, Doppler angle, and instrument settings on the accuracy of color Doppler ultrasound. Ultrasound Med Biol. 2001;27:551–64.
Kruskal JB, Newman PA, Sammons LG, et al. Optimizing Doppler and color flow US: application to hepatic sonography. Radiographics. 2004;24:657–75.
Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489–526.
Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:908-921.e6.
De Voogd F, Wilkens R, Gecse K, et al. A reliability study: strong inter-observer agreement of an expert panel for intestinal ultrasound in ulcerative colitis. J Crohns Colitis. 2021;15:1284–90.
Maaser C, Petersen F, Helwig U, et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut. 2020;69:1629–36.
Vuitton L, Peyrin-Biroulet L, Colombel JF, et al. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017;45:801–13.
Acknowledgements
This work was supported in part by grants from the Japan Sciences Research Grant for Research on Intractable Diseases (Japanese Inflammatory Bowel Disease Research Group) affiliated with the Japan Ministry of Health, Labour and Welfare. We thank Angela Morben, DVM, ELS, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
JM, RO, and HY conceived the study, designed the experiments, and analyzed the data. JM, RO, HY, HM, and NK prepared the manuscript. MM and TH supervised the writing of the manuscript. TH oversaw the entire project.
Corresponding author
Ethics declarations
Conflict of interest
Minoru Matsuura received consulting and lecture fees from Janssen Pharmaceutical K.K.; and received a scholarship grant from Nippon Kayaku Co., Ltd. Tadakazu Hisamatsu received consulting and lecture fees from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; and received research grants from Alfresa Pharma Co., Ltd., EA pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, JIMRO Co., Ltd., Zeria Pharmaceutical Co., Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Pfizer Inc., and Mochida Pharmaceutical Co., Ltd. Jun Miyoshi, Ryo Ozaki, Hiromi Yonezawa, Hideaki Mori, and Naohiro Kawamura have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyoshi, J., Ozaki, R., Yonezawa, H. et al. Ratio of submucosal thickness to total bowel wall thickness as a new sonographic parameter to estimate endoscopic remission of ulcerative colitis. J Gastroenterol 57, 82–89 (2022). https://doi.org/10.1007/s00535-021-01847-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-021-01847-3