Abstract
Background
Germline inactivating variants in the CDH1 tumor suppressor gene impart an elevated lifetime risk of diffuse gastric cancer. The current endoscopic surveillance method depends upon random gastric biopsies for early cancer detection.
Methods
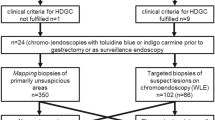
Asymptomatic adults with pathogenic or likely pathogenic CDH1 variants referred for endoscopic gastric cancer surveillance were included in this retrospective cohort. Upper gastrointestinal endoscopy was performed according to the consensus Cambridge method, in the early period, or a systematic (Bethesda) protocol as part of an ongoing natural history study. The primary outcome measure was cancer detection.
Results
Collectively, 135 endoscopic surveillance procedures were performed in 120 patients. Twenty-six (19%, 26/135) procedures were performed using Cambridge method and 109 (81%) using the Bethesda protocol. Gastric signet ring cell carcinomas were detected in 15% (4/26) using the Cambridge method and 36% (40/109) using the Bethesda protocol (p < 0.05). Almost half (44.2%, 53/120) of patients later elected for prophylactic total gastrectomy, of whom 51 (96%, 51/53) had a signet ring cell carcinoma (T1a) discovered by histopathology. On a per endoscopy basis, the false-negative rates of detection using Cambridge method and Bethesda protocol were 80% (12/15) and 37.7% (17/45), respectively (p < 0.01).
Conclusions
Gastric cancer detection was more frequent with implementation of a systematic surveillance protocol in CDH1 variant carriers. Given the decision for prophylactic surgery is often made by patients in the context of family history and pathologic result of surveillance biopsies, we propose the Bethesda protocol offers patients an opportunity to make more informed decisions.


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Palli D, Galli M, Caporaso NE, et al. Family history and risk of stomach cancer in Italy. Cancer Epidemiol Biomarkers Prev. 1994;3:15–8.
Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–5.
Berx G, Becker KF, Hofler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226–37.
Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60-70.
Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1:23–32.
Roberts ME, Ranola JMO, Marshall ML, et al. Comparison of CDH1 penetrance estimates in clinically ascertained families vs families ascertained for multiple gastric cancers. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.1208.
van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–74.
Xicola RM, Li S, Rodriguez N, et al. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet. 2019;56:838–43.
Lim YC, di Pietro M, O’Donovan M, et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest Endosc. 2014;80:78–87.
Mi EZ, Mi EZ, di Pietro M, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc. 2018;87:408–18.
Moreira L, Castells A. Surveillance of patients with hereditary gastrointestinal cancer syndromes. Best Pract Res Clin Gastroenterol. 2016;30:923–35.
Moslim MA, Heald B, Tu C, et al. Early genetic counseling and detection of CDH1 mutation in asymptomatic carriers improves survival in hereditary diffuse gastric cancer. Surgery. 2018;164:754–9.
Chen Y, Kingham K, Ford JM, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol. 2011;18:2594–8.
Hebbard PC, Macmillan A, Huntsman D, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol. 2009;16:1890–5.
Pandalai PK, Lauwers GY, Chung DC, et al. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery. 2011;149:347–55.
Kumar S, Katona BW, Long JM, et al. Endoscopic ultrasound has limited utility in diagnosis of gastric cancer in carriers of CDH1 mutations. Clin Gastroenterol Hepatol. 2020;18(505–508):e501.
Shaw D, Blair V, Framp A, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut. 2005;54:461–8.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013;26:11–22.
Garland SN, Lounsberry J, Pelletier G, Bathe OF. How do you live without a stomach? A multiple case study examination of total gastrectomy for palliation or prophylaxis. Palliat Support Care. 2011;9:305–13.
Hamilton JG, Long JM, Brandt AC, et al. Patients’ medical and psychosocial experiences after detection of a CDH1 variant with multigene panel testing. JCO Precis Oncol. 2019;3:1–14.
Fujita H, Lennerz JK, Chung DC, et al. Endoscopic surveillance of patients with hereditary diffuse gastric cancer: biopsy recommendations after topographic distribution of cancer foci in a series of 10 CDH1-mutated gastrectomies. Am J Surg Pathol. 2012;36:1709–17.
de Almeida Artifon EL, Marinho FRT. Endoscopic screening for hereditary diffuse gastric cancer: one size does not fit all. Gastrointest Endosc. 2018;87:405–7.
Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–87.
Barber ME, Save V, Carneiro F, et al. Histopathological and molecular analysis of gastrectomy specimens from hereditary diffuse gastric cancer patients has implications for endoscopic surveillance of individuals at risk. J Pathol. 2008;216:286–94.
Charlton A, Blair V, Shaw D, et al. Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut. 2004;53:814–20.
Ruff S, Curtin B, Quezado M, et al. Evaluation of confocal endoscopic microscopy for detection of early-stage gastric cancer in hereditary diffuse gastric cancer (HDGC) syndrome. J Gastrointest Oncol. 2019;10:407–11.
Funding
This study was supported in part by the Intramural Research Program, National Institutes of Health, National Cancer Institute.
Author information
Authors and Affiliations
Contributions
All the authors have contributed to and approved the final version of the manuscript. The respective roles of each author are the following: BFC, TH, CK, and JLD: study concept and design; all the authors: data analysis; all the authors: interpretation of results; BFC, LAG, SAS, and SMR: drafting the manuscript; all the authors: critical revision of the manuscript for valuable intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The authors have no relevant personal, professional or financial disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Curtin, B.F., Gamble, L.A., Schueler, S.A. et al. Enhanced endoscopic detection of occult gastric cancer in carriers of pathogenic CDH1 variants. J Gastroenterol 56, 139–146 (2021). https://doi.org/10.1007/s00535-020-01749-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-020-01749-w