Abstract
Background
Helicobacter pylori causes peptic ulcers and accounts for over 90% of gastric cancers; however, eradication rates have been declining due to antimicrobial resistance. Vonoprazan (VPZ), a potassium-competitive acid blocker, produces rapid and profound gastric acid suppression and has shown promising effects in the improvement of H. pylori eradication rates. The efficacy and safety of VPZ-based triple therapy as a first-line regimen for H. pylori eradication and its relationship with clarithromycin (CAM) susceptibility were evaluated.
Methods
From May 2015 to September 2017, H. pylori-infected patients who underwent esophagogastroduodenoscopy with CAM susceptibility testing were prospectively enrolled. Patients received a 7-day triple therapy regimen (VAC) of VPZ (20 mg), amoxicillin (750 mg), and CAM (200 mg) twice daily. Eradication rates, demographics, CAM susceptibility, and safety profiles were assessed.
Results
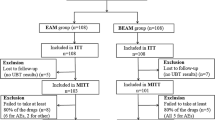
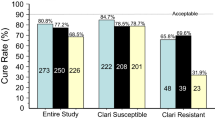
VAC was administered to 146 patients (median age: 63, range: 22–85 years) (60% of whom were females) who underwent CAM susceptibility testing, and 131 patients underwent 13C-urea breath testing to evaluate eradication success. The prevalence of CAM resistance was 34.2%. The overall eradication rates of VAC in per protocol (PP) and “intention to treat” (ITT) analyses were 90.8% (n = 131) and 81.5% (n = 146), respectively. In PP analysis for CAM susceptibility, the eradication rates of VAC were comparable between CAM-sensitive (91.6%, n = 83) and CAM-resistant (89.4%, n = 47) strains. The corresponding rates from the ITT analysis were 80.0% (n = 95) and 84.0% (n = 50), respectively. No adverse events requiring discontinuation of VAC were observed.
Conclusions
CAM-resistant H. pylori was prevalent in one-third of patients in the Tokyo metropolitan area. VPZ-based triple therapy was highly effective and well-tolerated irrespective of CAM susceptibility. Therefore, it could be a valuable first-line treatment regimen for H. pylori infection.


Similar content being viewed by others
References
Moss SF. The clinical evidence linking Helicobacter pylori to gastric cancer. Cell Mol Gastroenterol Hepatol. 2016;3:183–91.
Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol. 2017;14:383–4.
Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment PharmacolTher. 2016;43:514–33.
Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–67.
Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: current status and future concepts. World J Gastroenterol. 2014;20:5283–93.
Noda H, Noguchi S, Yoshimine T, et al. A novel potassium-competitive acid blocker improves the efficacy of clarithromycin-containing 7-day triple therapy against Helicobacter pylori. J Gastrointestin Liver Dis. 2016;25:283–8.
Matsumoto H, Shiotani A, Katsumata R, et al. Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: the effect of clarithromycin resistance. Dig Dis Sci. 2016;61:3215–20.
Akazawa Y, Fukuda D, Fukuda Y. Vonoprazan-based therapy for Helicobacter pylori eradication: experience and clinical evidence. Therap Adv Gastroenterol. 2016;9:845–52.
Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335:231–8.
Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–46.
Morimoto N, Takeuchi H, Nishida Y, et al. Clinical application of the DiversiLab microbial typing system using repetitive sequence-based PCR for characterization of Helicobacter pylori in Japan. J Clin Lab Anal. 2015;29:250–3.
Sasaki M, Ogasawara N, Utsumi K, et al. Changes in 12-year first-line eradication rate of Helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J Clin BiochemNutr. 2010;47:53–8.
Tsujimae M, Yamashita H, Hashimura H, et al. A comparative study of a new class of gastric acid suppressant agent named vonoparazan versus esomeprazole for the eradication of Helicobacter pylori. Digestion. 2016;94:240–6.
Sakurai K, Suda H, Ido Y, et al. Comparative study: vonoprazan and proton pump inhibitors in Helicobacter pylori eradication therapy. World J Gastroenterol. 2017;23:668–75.
Shichijo S, Hirata Y, Niikura R, et al. Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: a multicenter retrospective study in clinical practice. J Dig Dis. 2016;17:670–5.
Sue S, Kuwashima H, Iwata Y, et al. The superiority of vonoprazan-based first-line triple therapy with clarithromycin: a prospective multi-center cohort study on Helicobacter pylori eradication. Intern Med. 2017;56:1277–85.
Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment PharmacolTher. 2017;46:106–14.
Hunt RH, Scarpignato C. Potassium-competitive acid blockers (P-CABs): are they finally ready for prime time in acid-related disease? Clin Transl Gastroenterol. 2015;6:e119.
Calvet X, Gomollon F. What is potent acid inhibition, and how can it be achieved? Drugs. 2005;65:13–23.
Sachs G, Scott DR, Wen Y. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep. 2011;13:540–6.
Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–31.
Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: Vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment PharmacolTher. 2016;43:240–51.
Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of vonoprazan for Helicobacter pylori eradication. Intern Med. 2020;59:153–61.
Mori H, Suzuki H, Omata F, et al. Current status of first- and second-line Helicobacter pylori eradication therapy in the metropolitan area: a multicenter study with a large number of patients. Therap Adv Gastroenterol. 2019;12:1756284819858511.
Perri F, Villani MR, Festa V, et al. Predictors of failure of Helicobacter pylori eradication with the standard ‘maastricht triple therapy’. Aliment PharmacolTher. 2001;15:1023–9.
Poon SK, Chang CS, Su J, et al. Primary resistance to antibiotics and its clinical impact on the efficacy of Helicobacter pylori lansoprazole-based triple therapies. Aliment PharmacolTher. 2002;16:291–6.
Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the maastricht V/florence consensus report. Gut. 2017;66:6–30.
Horiki N, Omata F, Uemura M, et al. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009;14:86–90.
Perez Aldana L, Kato M, Nakagawa S, et al. The relationship between consumption of antimicrobial agents and the prevalence of primary Helicobacter pylori resistance. Helicobacter. 2002;7:306–9.
Sue S, Ogushi M, Arima I, et al. Vonoprazan-vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: a multicenter, prospective, randomized trial. Helicobacter. 2018;23:e12456.
Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743–9.
Furuta T, Yamade M, Kagami T, et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion. 2019. https://doi.org/10.1159/000502287.
Midolo PD, Turnidge JD, Munckhof WJ. Is bactericidal activity of amoxicillin against Helicobacter pylori concentration dependent? Antimicrob Agents Chemother. 1996;40:1327–8.
Kalkan IH, Sapmaz F, Guliter S, Atasoy P. Severe gastritis decreases success rate of Helicobacter pylori eradication. Wien KlinWochenschr. 2016;128:329–34.
Baena JM, Lopez C, Hidalgo A, et al. Relation between alcohol consumption and the success of Helicobacter pylori eradication therapy using omeprazole, clarithromycin and amoxicillin for 1 week. Eur J Gastroenterol Hepatol. 2002;14:291–6.
Takara Y, Endo H, Nakano R, et al. Smoking and drinking did not increase the failure of therapeutic Helicobacter pylori eradication by vonoprazan, clarithromycin, and amoxicillin. Digestion. 2019;99:172–8.
Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–24.
Suzuki S, Gotoda T, Kusano C, et al. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol. 2016;111:949–56.
Dong SQ, Singh TP, Wei X, et al. Review: a Japanese population-based meta-analysis of vonoprazan versus PPI for Helicobacter pylori eradication therapy: Is superiority an illusion? Helicobacter. 2017;22:e12438. https://doi.org/10.1111/hel.12438.
Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentrerandomised trial in Japan. Gut. 2020;69:1019–26.
Okamura T, Suga T, Nagaya T, et al. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter. 2014;19:214–20.
Su P, Li Y, Li H, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18:274–9.
Zhang YX, Zhou LY, Song ZQ, et al. Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: a prospective serial study. World J Gastroenterol. 2015;21:2786–92.
Acknowledgments
We thank Kazuhisa Mezaki, the clinical microbiological technician, who cultured the Helicobacter pylori.
Author information
Authors and Affiliations
Contributions
Hidetaka Okubo, Junichi Akiyama, Masao Kobayakawa, Megumi Kawazoe, Saori Mishima, Yuya Takasaki, Naoyoshi Nagata, Takayuki Shimada, Chizu Yokoi, Shiori Moriyasu, Kana Kimura, Yuya Hisada, Eri Iwata, Kazuhiro Watanabe, Naohiro Yanagisawa, Shou Shiroma, Akira Shimomura, Kouki Okahara, and Hourin Cho collected data. Hidetaka Okubo designed the study and wrote the initial draft of the manuscript. Junichi Akiyama and Naomi Uemura contributed to analysis and interpretation of data and assisted in the preparation of the manuscript. All authors approved the final version of the manuscript, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interests
Junichi Akiyama has served as a speaker for Takeda Pharmaceutical Company. Naomi Uemura has served as a speaker for Takeda Pharmaceutical Company and Astra Zeneca Company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okubo, H., Akiyama, J., Kobayakawa, M. et al. Vonoprazan-based triple therapy is effective for Helicobacter pylori eradication irrespective of clarithromycin susceptibility. J Gastroenterol 55, 1054–1061 (2020). https://doi.org/10.1007/s00535-020-01723-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-020-01723-6