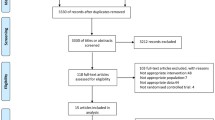
Abstract
Background
Although indigo naturalis (IN) is effective for patients with active ulcerative colitis (UC), IN was associated with adverse events (AEs), including pulmonary arterial hypertension (PAH). Our aim was to evaluate the occurrence of IN-associated AEs and to evaluate any IN dose–effect on AEs.
Methods
A nationwide survey, using questionnaires, was conducted by conducted by the research group funded by the Ministry of Health, Labour and Welfare of Japan, between June 2017 and September 2018. A first questionnaire determined the occurrence of AEs associated with the therapeutic use of IN or herbal medicines containing IN in patients with UC. A second survey identified the clinical characteristics of patients who developed IN-associated critical AEs, namely, liver dysfunction, PAH, and intussusception.
Results
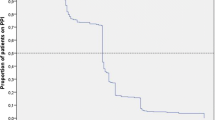
Across 337 participating institutions, 49,320 patients with UC were identified, with IN used in 877 (1.8%). AEs were reported in 91 patients (107 events), including liver dysfunction (n = 40), gastrointestinal symptoms (n = 21), headache (n = 13), and PAH (n = 11). No dose–effect relationship between IN and AEs was identified. Liver dysfunction tended to be mild and reversible. Ten cases of intussusception were reported, with 40% of these patients requiring surgical resection. IN-induced PAH was recovered in patients who discontinued to use IN. No IN-associated deaths were reported.
Conclusions
IN-associated AEs were identified among patients with UC, with liver dysfunction often being reversible, while surgical resection was required in a high proportion of patients who developed intussusception. Both healthcare workers and patients should adequately recognize the potential for AEs with the use of IN.
Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- γ-GTP:
-
γ-Glutamyl transpeptidase
- IN:
-
Indigo naturalis
- PAH:
-
Pulmonary arterial hypertension
- UC:
-
Ulcerative colitis
References
Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51:853–61.
Naganuma M, Sugimoto S, Mitsuyama K, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154:935–47.
Nishio M, Hirooka K, Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur Heart J. 2016;37:1992.
Sugimoto S, Naganuma M, Kiyohara H, et al. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion. 2016;93:193–201.
Kondo S, Araki T, Okita Y, et al. Colitis with wall thickening and edematous changes during oral administration of the powdered form of Qing-dai in patients with ulcerative colitis: a report of two cases. Clin J Gastroenterol. 2018;11:268–72.
Yanai S, Nakamura S, Matsumoto T. Indigo naturalis-induced colitis. Dig Endosc. 2018;30:791.
Zhang ZM, Lin XC, Ma L, et al. Ischemic or toxic injury: a challenging diagnosis and treatment of drug-induced stenosis of the sigmoid colon. World J Gastroenterol. 2017;23:3934–44.
Matsuno Y, Hirano A, Esaki M. Possible association of phlebitis-induced colitis with indigo naturalis. Gastroenterology. 2018;155:576–7.
Marinis A, Yiallourou A, Samanides L, et al. Intussusception of the bowel in adults: a review. World J Gastroenterol. 2009;15:407–11.
Begos DG, Sandor A, Modlin IM. The diagnosis and management of adult intussusception. Am J Surg. 1997;173:88–94.
Choi YS, Suh JP, Lee IT, et al. Regression of giant pseudopolyps in inflammatory bowel disease. J Crohns Colitis. 2012;6:240–3.
Esaki M, Matsumoto T, Fuyuno Y, et al. Giant inflammatory polyposis of the cecum with repeated intussusception in ulcerative colitis: report of a case. Am J Gastroenterol. 2009;104:2873–4.
Maldonado TS, Firoozi B, Stone D, et al. Colocolonic intussusception of a giant pseudopolyp in a patient with ulcerative colitis: a case report and review of the literature. Inflamm Bowel Dis. 2004;10:41–4.
Takeuchi K, Tsuzuki Y, Ando T, et al. The diagnosis and treatment of adult intussusception. J Clin Gastroenterol. 2003;36:18–211.
Nishio M, Hirooka K, Doi Y. Pulmonary arterial hypertension associated with the Chinese herb indigo naturalis for ulcerative colitis: it may be reversible. Gastroenterology. 2018;155:577–8.
Lin YK, See LC, Huang YH, et al. Comparison of indirubin concentrations in indigo naturalis ointment for psoriasis treatment: a randomized, double-blind, dosage-controlled trial. Br J Dermatol. 2018;178:124–31.
Acknowledgements
We thank all the collaborators who cooperated in this first nationwide survey of IN-induced adverse events in Japan. INDIGO survey Group are Makoto Naganuma, Shinya Sugimoto, Tomohiro Fukuda, Yusuke Yoshimatsu, Po-sung Chu, Nobuhiro Nakamoto, Masaharu Kataoka, Takanori Kanai (Keio University School of Medicine), Toshifumi Hibi (Kitasato Institute Hospital), Hideki Iijima (Osaka University Graduate School of Medicine), Motoi Uchino, Kenji Watanabe, Shiro Nakamura (Hyogo College of Medicine), Yasuo Suzuki (Toho University Sakura Medical Center), Yuichi Matsuno, Atsushi Hirano, Motohiro Esaki (Kyushu University), Ryohei Hayashi, Yoshitaka Ueno (Hiroshima University), Takayuki Yamamoto (Yokkaichi Hazu Medical Center), Koji Sawada (Dojima General and Gastroenterology Clinic), Kosaku Kawashima, Shunji Ishihara (Shimane University), Toshimitsu Araki (Mie University), Shinichi Hashimoto (Yamaguchi University), Junji Yokoyama (Niigata University), Shinya Ashizuka (University of Miyazaki), Hideo Suzuki (Tsukuba University), Tatsuya Toyokawa (Fukuyama Medical Center), Hirotake Sakuraba (Hirosaki University), Kazuhiro Matsueda (Kurashiki Central Hospital), Naoki Yoshimura (Yamate Medical Center), Naoki Ohmiya (Fujita Health University School of Medicine), Yoshiyuki Kayashima (Matsuyama Red Cross Hospital), Shunichi Yanai, Takayuki Matsumoto (Iwate Medical University), Hiroyuki Kobayashi (Fukuoka Sanno Hospital), Sachiko Nakajima, Tsutomu Nishida (Toyonaka Municipal Hospital), Yoshiaki Takeuchi (Showa University), Hirotaka Shimizu (National Center for Child Health and Development), Kenji Ina (Nagoya Memorial Hospital), Takuji Kawamura (Japanese Red Cross Kyoto Daini Hospital).
Funding
This study was supported in part by Research Grants for special research on intractable diseases from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Consortia
Contributions
TK conceived the study. MN designed the main concept of this study and drafted the main protocol. SS and NN helped to draft the main protocol. MN and SS participated in analysis and interpretation of data. All the authors participated in patient enrolment and clinical data acquisition. MN, SS and TK drafted and wrote the manuscript. All the authors contributed to critical review and approved the final draft.
Corresponding authors
Ethics declarations
Conflict of interest
MN received commercial research funds from EA pharma Co., Ltd. and Mochida Pharmaceutical Co., Ltd., outside the submitted work. SN received lecture fees from Mitsubishi Tanabe Pharma Corp., AbbVie GK, Zeria Pharmaceutical co., Ltd., EA pharma Co., Ltd., and commercial research funds from Mitsubishi Tanabe Pharma Corp., AbbVie GK, EA pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd, JIMRO Co., Ltd., Otsuka Pharmaceutical Factory Inc., Takeda Pharmaceutical Co., Ltd, outside the submitted work. YS received lecture fees from Mitsubishi Tanabe Pharma Corp., AbbVie GK, Zeria Pharmaceutical co., Ltd., EA pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd. Janssen Japan Co., Ltd and commercial research funds from Mitsubishi Tanabe Pharma Corp., EA pharma Co., Ltd., AbbVie GK, EA pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd, AbbVie GK, JIMRO Co., Ltd., outside the submitted work. TK received lecture fees from Mitsubishi Tanabe Pharma Corp, Astellas Pharma Inc, Miyarisan Pharmaceutical Co., Ltd. AstraZeneca Plc, EA Pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd. and commercial research funds from AbbVie GK, Mochida Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corp, Takeda Pharmaceutical Co., Ltd., Nihon Kayaku, Yakult Honsha Co., Ltd., Zeria Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., Ezaki Glico Co., Ltd. JIMRO Co., Ltd., EN Otsuka Pharmaceutical Co., Ltd, outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
INDIGO survey Group members are listed in the Acknowledgements section.
Rights and permissions
About this article
Cite this article
Naganuma, M., Sugimoto, S., Suzuki, H. et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol 54, 891–896 (2019). https://doi.org/10.1007/s00535-019-01591-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01591-9