Abstract
Background
Glecaprevir and pibrentasvir (GLE/PIB) are potent antiviral agents for hepatitis C virus (HCV) pan-genotypic infections; however, their clinical effectiveness and safety remain limited in the real-world. This study aimed to evaluate viral responses and the safety of GLE/PIB for patients with chronic HCV-1/2/3 infections during both initial- (Arm A) and re-treatment (Arm B) with all-oral direct-acting antiviral agents (DAAs).
Methods
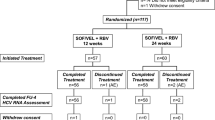
This prospective-observational cohort study included Japanese patients with chronic HCV-1/2/3 infections (n = 271: 183 in Arm A and 83 in Arm B), who had started receiving GLE/PIB. Primary end point was a sustained virological response (SVR) rate at week 12 (SVR12) after the end of GLE/PIB treatment (EOT).
Results
SVR12 was achieved by 99.4% of patients (180/181: modified intention-to-treat (mITT) analysis excluding 2 patients lost to follow-up) in Arm A. One patient with an HCV-3b infection who discontinued at week 8 failed to achieve SVR12. SVR12 was achieved by 97.7% of patients (85/87: mITT excluding 1 patient lost to follow-up) in Arm B. Virological relapse occurred in 2 patients with HCV-1b, presenting common 5 loci of resistance-associated substitutions (RASs) including A92 RASs in the NS5A lesion at baseline. Any adverse events (AEs) (grade ≥ 3) occurred in 8 patients (3.0%). 8 patients (3.0%) discontinued due to AEs, however, all of them achieved SVR12.
Conclusions
Initial and re-treatment with GLE/PIB are effective and safe for Japanese patients with HCV-1/2/3 in real-life settings. Further studies are required to elucidate the mechanism underlying treatment failures of GLE/PIB to completely eradicate HCV worldwide.





Similar content being viewed by others
Abbreviations
- DAAs:
-
Direct-acting antiviral agents
- HCV:
-
Hepatitis C virus
- SVR:
-
Sustained virological response
- IFN:
-
Interferon
- DCV:
-
Daclatasvir
- ASV:
-
Asunaprevir
- GT:
-
Genotype
- SOF:
-
Sofosbuvir
- LDV:
-
Ledipasvir
- PrO:
-
Paritaprevir/ritonavir plus ombitasvir
- EBR:
-
Elbasvir
- GZR:
-
Grazoprevir
- DCV-TRIO:
-
DCV/ASV plus beclabuvir
- RBV:
-
Ribavirin
- NS:
-
Non-structural protein
- GLE:
-
Glecaprevir
- PIB:
-
Pibrentasvir
- RASs:
-
Resistance-associated substitutions
- ITT:
-
Intention-to-treat
- HCC:
-
Hepatocellular carcinoma
- EOT:
-
End of treatment
- LLOQ:
-
Lower limit of quantification
- eGFR:
-
Estimated glomerular filtration rate
- CKD:
-
Chronic kidney disease
- ALT:
-
Alanine aminotransferase
- AFP:
-
Alpha-fetoprotein
- CI:
-
Confidence interval
- VEL:
-
Velpatasvir
- VOX:
-
Voxilaprevir
References
Manns P, Buti M, Gane E, Pawlotsky JM, et al. Hepatitis C virus infection. Nat Rev Dis Primers. 2017;3:17006.
Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–91.
Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645–53.
Kumada H, Chayama K, Rodrigues L Jr, et al. Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology. 2015;62:1037–46.
Kumada H, Suzuki Y, Karino Y, et al. The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients: a randomized phase II/III study. J Gastroenterol. 2017;52:520–33.
Toyota J, Karino Y, Suzuki F, et al. Daclatasvir/asunaprevir/beclabuvir fixed-dose combination in Japanese patients with HCV genotype 1 infection. J Gastroenterol. 2017;52:385–95.
Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762–8.
Sato K, Chayama K, Alves K, et al. Randomized phase 3 Trial of ombitasvir/paritaprevir/ritonavir and ribavirin for hepatitis C virus genotype 2-infected Japanese patients. Adv Ther. 2017;34:1449–65.
Terrault NA, Zeuzem S, Di Bisceglie AM, et al. Effectiveness of ledipasvir–sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151:1131–40.
Sezaki H, Suzuki F, Hosaka T, et al. The efficacy and safety of dual oral therapy with daclatasvir and asunaprevir for genotype 1b in Japanese real-life settings. Liver Int. 2017;37:1325–33.
Akuta N, Sezaki H, Suzuki F, et al. Ledipasvir plus sofosbuvir as salvage therapy for HCV genotype 1 failures to prior NS5A inhibitors regimens. J Med Virol. 2017;89:1248–54.
Suda G, Ogawa K, Yamamoto Y, et al. Retreatment with sofosbuvir, ledipasvir, and add-on ribavirin for patients who failed daclatasvir and asunaprevir combination therapy. J Gastroenterol. 2017;52:1122–9.
Iio E, Shimada N, Takaguchi K, et al. Clinical evaluation of sofosbuvir/ledipasvir in patients with chronic hepatitis C genotype 1 with and without prior daclatasvir/asunaprevir therapy. Hepatol Res. 2017;52:1122–9.
Poordad F, Felizarta F, Asatryan A, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017;66:389–97.
Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17:1062–8.
Zeuzem S, Foster GR, Wang S, et al. Glecaprevir–pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378:354–69.
Chayama K, Suzuki F, Karino Y, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis. J Gastroenterol. 2018;53:557–65.
Toyada H, Chayama K, Suzuki F, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection. Hepatology. 2018;67:505–13.
Kumada H, Watanabe T, Suzuki F, et al. Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol. 2018;53:566–75.
Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis C Virus Infection. A 2017 update (Japanese).
Ogasawara N, Kobayashi M, Akuta N, et al. Serial changes in liver stiffness and controlled attenuation parameter following direct-acting antiviral therapy against hepatitis C virus genotype 1b. J Med Virol. 2018;90:313–9.
Ogura S, Saitho S, Kawamura Y, et al. Magnetic resonance laparoscopy: a new non-invasive technique for the assessment of chronic viral liver disease. Hepatol Res. 2013;43:836–45.
Kidney Disease Improving Global Outcomes (KDIGO). KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:S1–50.
Kato N, Hijikata M, Ootsuyama Y, et al. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci USA. 1990;87:9524–8.
McPhee F, Hernandez D, Zhou N, et al. Virologic escape in HCV genotype 1-infected patients receiving daclatasvir plus ribavirin and peginterferon alfa-2a or alfa-2b. Antivir Ther. 2014;19:479–90.
Pawlotsky JM. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology. 2016;151:70–86.
Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64:486–504.
Akuta N, Sezaki H, Suzuki F, et al. Favorable efficacy of glecaprevir plus pibrentasvir as salvage therapy for HCV failures to prior direct-acting antivirals regimens. J Med Virol. 2019;91:102–6.
American Association for the Study of Liver disease (AASLD), Infectious Disease Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. https://www.hcvguidelines.org/.
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
D’Ambrosio R, Pasulo L, Puoti M, et al. Real-life effectiveness and safety of glecaprevir/pibrentasvir among 723 Italian patients with chronic hepatitis C: the navigator-II study. J Hepatol. 2018 [Epub ahead of print].
Krishnan P, Schnell G, Tripathi R, et al. Integrated resistance analysis of CERTAIN-1 and CERTAIN-2 studies in hepatitis C virus-infected patients receiving glecaprevir and pibrentasvir in Japan. Antimicrob Agents Chemother. 2018;62:e02217.
Tellinghuisen TL, Marcotrigiano J, Rice CM. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature. 2005;435:374–9.
Love RA, Brodsky O, Hickey MJ, Wells PA, Cronin CN. Crystal structure of a novel dimeric form of NS5A domain I protein from hepatitis C virus. J Virol. 2009;83:4395–403.
Ascher DB, Wielens J, Nero TL, Doughty L, Marton CJ, Parker MW. Potent hepatitis C inhibitors bind directly to NS5A and reduce its affinity for RNA. Sci Rep. 2014;4:04765.
Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–9.
Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801.
Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164–71.
Meissner E, Bon D, Prokunina-Olsson P, et al. IFNL4-ΔG genotype is associated with slower viral clearance in hepatitis C, genotype-1 patients treated with sofosbuvir and ribavirin. J Infect Dis. 2014;209:1700–4.
Osawa M, Imamura M, Teraoka Y, et al. Real-world efficacy of glecaprevir plus pibrentasvir for chronic hepatitis C patient with previous direct-acting antiviral therapy failures. J Gastroenterol. 2018 [Epub ahead of print].
Bourlière M, Gordon SC, Schiff ER, et al. Deferred treatment with sofosbuvir-velpatasvir-voxilaprevir for patients with chronic hepatitis C virus who were previously treated with an NS5A inhibitor: an open-label substudy of POLARIS-1. Lancet Gastroenterol Hepatol. 2018;3:559–65.
Izumi N, Takehara T, Chayama K, et al. Sofosbuvir–velpatasvir plus ribavirin in Japanese patients with genotype 1 or 2 hepatitis C who failed direct-acting antivirals. Hepatol Int. 2018;12:356–67.
Asselah T, Kowdley KV, Zadekis N, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018;16:417–26.
Acknowledgements
This work was partly supported by health sciences research grants from the Ministry of Health, Labor, and Welfare of Japan and AMED (Japan Agency for Medical Research and Development; Research on Hepatitis, grant number: 18fk0210002h0003). We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
HS: Study concept and design; data collection, analysis, and interpretation; and drafting of the manuscript. FS: Data collection, analysis, and interpretation and review of the manuscript. TH: Data collection, analysis, interpretation and review of the manuscript, and drafting of the manuscript. SF: Data collection and review of the manuscript. YK: Data collection and review of the manuscript. NA: Data collection and review of the manuscript. MK: Data collection and review of the manuscript. YS: Data collection and review of the manuscript. SS: Data collection and review of the manuscript. YA: Data collection and review of the manuscript. KI: Data collection and review of the manuscript. MK: Direct sequencing HCV genome, genotyping IL28B polymorphism, assistance with data collection, and review of the manuscript. HK: Study concept and design; data collection, analysis, and interpretation; and critical review of the manuscript
Corresponding author
Ethics declarations
Conflict of interest
Hiromitsu Kumada received honoraria for lectures from MSD K.K., Bristol-Myers Squibb, Gilead Sciences, AbbVie Inc., Glaxo Smith Klein K.K. and Dainippon Sumitomo Pharma. Fumitaka Suzuki received honoraria for lectures from Bristol-Myers Squibb and AbbVie Inc. Yoshiyuki Suzuki received honoraria for lectures from Bristol-Myers Squibb and AbbVie Inc. Kenji Ikeda received honoraria for lectures from Dainippon Sumitomo Pharma, Eisai Co., Ltd. Norio Akuta received honoraria for lectures from Bristol-Myers Squibb and AbbVie Inc. The other authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sezaki, H., Suzuki, F., Hosaka, T. et al. Initial- and re-treatment effectiveness of glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C virus-genotype 1/2/3 infections. J Gastroenterol 54, 916–927 (2019). https://doi.org/10.1007/s00535-019-01575-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01575-9