Abstract
Background
The mechanism behind the pathogenesis and carcinogenesis of these neoplasms is not fully understood. The objective of this study was to identify genetic markers and pathways specific to precancerous duodenal adenomas and early stage adenocarcinomas through gene expression analysis.
Methods
Gene expression profiling was performed in 4 pairs of duodenal adenoma/adenocarcinomas and corresponding matched normal tissue. Genes with consistent expression differences were identified and confirmed in 7 independent pairs. Gene set enrichment analysis (GSEA) was performed to characterize gene expression profiles of duodenal adenoma/adenocarcinomas, together with immunohistochemical staining of candidate oncogenic genes.
Results
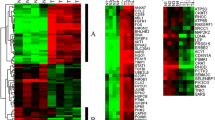
626 probes consistently demonstrated over a twofold expression difference between tumor–normal pairs. Reverse transcriptase polymerase chain reaction of genes with the most prominent difference in expression between tumors and normal mucosa (KLK7, KLK6, CEMIP, MMP7, KRT17, LGR5, G6PC, S100G, APOA1) validated the results of gene expression analysis. GSEA demonstrated a strong association between duodenal adenoma/adenocarcinomas with colorectal adenomas (p < 10−5) and gene expression patterns seen after APC gene knockout (p < 10−5), suggesting that the Wnt/β-catenin pathway plays a crucial role in the carcinogenesis of these neoplasms. Immunohistochemical staining of an independent group of duodenal adenomas confirmed over-accumulation of β-catenin in 80.0% (16/20).
Conclusions
Precancerous duodenal adenomas and early stage adenocarcinomas demonstrate gene expression characteristics with a strong resemblance to colorectal adenomas. The results of this study strongly suggest that upregulation of the Wnt/β-catenin pathway is the major factor involved in the initial stages of the carcinogenesis of duodenal adenocarcinomas.





Similar content being viewed by others

References
Raghav K, Overman MJ. Small bowel adenocarcinomas—existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10:534–44.
Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518–26.
Culver EL, McIntyre AS. Sporadic duodenal polyps: classification, investigation, and management. Endoscopy. 2011;43:144–55.
Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48:799–819.
Gold JS, Tang LH, Gönen M, et al. Utility of a prognostic nomogram designed for gastric cancer in predicting outcome of patients with R0 resected duodenal adenocarcinoma. Ann Surg Oncol. 2007;14:3159–67.
Hida R, Yamamoto H, Hirahashi M, et al. Duodenal neoplasms of gastric phenotype: an immunohistochemical and genetic study with a practical approach to the classification. Am J Surg Pathol. 2017;41:343–53.
Matsubara A, Ogawa R, Suzuki H, et al. Activating GNAS and KRAS mutations in gastric foveolar metaplasia, gastric heterotopia, and adenocarcinoma of the duodenum. Br J Cancer. 2015;112:1398–404.
Ushiku T, Arnason T, Fukayama M, et al. Extra-ampullary duodenal adenocarcinoma. Am J Surg Pathol. 2014;38:1484–93.
Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2011;106:357–64.
Kakushima N, Kanemoto H, Sasaki K, et al. Endoscopic and biopsy diagnoses of superficial, nonampullary, duodenal adenocarcinomas. World J Gastroenterol. 2015;21:5560–7.
Kinoshita S, Nishizawa T, Ochiai Y, et al. Accuracy of biopsy for the preoperative diagnosis of superficial nonampullary duodenal adenocarcinoma. Gastrointest Endosc. 2017;86:329–32.
Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71.
Holec M, Kléma J, Zelezný F, et al. Comparative evaluation of set-level techniques in predictive classification of gene expression samples. BMC Bioinform. 2012;13(Suppl 10):S15.
Bateman AR, El-Hachem N, Beck AH, et al. Importance of collection in gene set enrichment analysis of drug response in cancer cell lines. Sci Rep. 2014;4:4092.
Brabletz T, Herrmann K, Jung A, et al. Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol. 2000;156:865–70.
Birkenkamp-Demtroder K, Maghnouj A, Mansilla F, et al. Repression of KIAA1199 attenuates Wnt-signalling and decreases the proliferation of colon cancer cells. Br J Cancer. 2011;105:552–61.
Sabates-Bellver J, Van der Flier LG, de Palo M, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–75.
Brabletz T, Jung A, Dag S, et al. Beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–8.
Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–91.
Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7.
van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–50.
Van der Flier LG, Sabates-Bellver J, Oving I, et al. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–32.
Johnson SK, Ramani VC, Hennings L, et al. Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer. 2007;109:1811–20.
Schuster R, Max N, Mann B, et al. Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer. 2004;108:219–27.
Tassi E, Henke RT, Bowden ET, et al. Expression of a fibroblast growth factor-binding protein during the development of adenocarcinoma of the pancreas and colon. Cancer Res. 2006;66:1191–8.
Nissan A, Stojadinovic A, Mitrani-Rosenbaum S, et al. Colon cancer associated transcript-1: a novel RNA expressed in malignant and pre-malignant human tissues. Int J Cancer. 2012;130:1598–606.
Sakamoto H, Mutoh H, Miura Y, et al. SOX9 is highly expressed in nonampullary duodenal adenoma and adenocarcinoma in humans. Gut Liver. 2013;7:513–8.
Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–90.
Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–91.
Yuan W, Zhang Z, Dai B, et al. Whole-exome sequencing of duodenal adenocarcinoma identifies recurrent Wnt/β-catenin signaling pathway mutations. Cancer. 2016;122:1689–96.
Wagner PL, Chen YT, Yantiss RK. Immunohistochemical and molecular features of sporadic and FAP-associated duodenal adenomas of the ampullary and nonampullary mucosa. Am J Surg Pathol. 2008;32:1388–95.
Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–61.
Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60.
Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600.
Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5.
Acknowledgements
The authors thank Shinya Kodashima, Satoshi Ono, Yosuke Tsuji, Keiko Niimi for their assistance as participating investigators in acquisition of samples, and Hironori Waki, Yuta Hiraike for their assistance in RNA extraction.
Funding
This work was supported in part by a research grant from the Japanese Foundation for Research and Promotion of Endoscopy, in part by Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science, and in part by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Conception and design: YS, NY, ST. Development of methodology: YS, NY, ST, CT, NKY, YT, KS, KI. Acquisition of data: YS, MI, MF. Analysis and interpretation of data: YS, NY, ST, CT, YT, KS, KI. Writing, review, and/or revision of the manuscript: YS, NY, ST, CT, NKY, YT, KK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2018_1489_MOESM2_ESM.jpg
Supplementary Figure 1: Localization of Over-accumulation of β-catenin. a) β-catenin in duodenal and colorectal adenomas was similarly over-expressed in both the cytoplasm (80.0% vs 86.7%) and nucleus (15.0% vs 20.0%). b) Representative cases of duodenal adenoma. A case with both cytoplasmic and nuclear over-expression of β-catenin (left). A case with only cytoplasmic over-expression of β-catenin with no nuclear staining (right). c) Representative cases of colorectal adenoma. A case with both cytoplasmic and nuclear over-expression of β-catenin (left). A case with only cytoplasmic over-expression of β-catenin with no nuclear staining (right) (JPEG 4840 kb)
535_2018_1489_MOESM3_ESM.jpg
Supplementary Figure 2: a) GSEA demonstrates that FAP-related tumors demonstrate enrichment in the Wnt/β-catenin pathway, with enrichment scores similar to sporadic duodenal tumors. b) The gene expression values of the top up-regulated genes in sporadic duodenal tumors. Only minimal changes can be seen in the gene expression values of these genes in FAP-related duodenal adenomas. c) The gene expression values of the top down-regulated genes in sporadic duodenal tumors. Similarly, only minimal changes can be seen in the gene expression values of these genes in FAP-related duodenal adenomas (JPEG 3758 kb)
Rights and permissions
About this article
Cite this article
Sakaguchi, Y., Yamamichi, N., Tomida, S. et al. Identification of marker genes and pathways specific to precancerous duodenal adenomas and early stage adenocarcinomas. J Gastroenterol 54, 131–140 (2019). https://doi.org/10.1007/s00535-018-1489-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1489-4