Abstract
Background
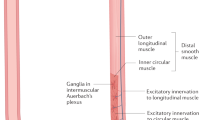
The existence of several autoantibodies suggests an autoimmune basis for gastrointestinal (GI) dysmotility. Whether GI motility disorders are features of autoimmune autonomic ganglionopathy (AAG) or are related to circulating anti-ganglionic acetylcholine receptor (gAChR) antibodies (Abs) is not known. The aim of this study was to determine the associations between autonomic dysfunction, anti-gAChR Abs, and clinical features in patients with GI motility disorders including achalasia and chronic intestinal pseudo-obstruction (CIPO).
Methods
First study: retrospective cohort study and laboratory investigation. Samples from 123 patients with seropositive AAG were obtained between 2012 and 2017. Second study: prospective study. Samples from 28 patients with achalasia and 14 patients with CIPO were obtained between 2014 and 2016, and 2013 and 2017, respectively. In the first study, we analyzed clinical profiles of seropositive AAG patients. In the second study, we compared clinical profiles, autonomic symptoms, and results of antibody screening between seropositive, seronegative achalasia, and CIPO groups.
Results
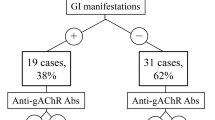
In the first study, we identified 10 patients (8.1%) who presented with achalasia, or gastroparesis, or paralytic ileus. In the second study, we detected anti-gAChR Abs in 21.4% of the achalasia patients, and in 50.0% of the CIPO patients. Although patients with achalasia and CIPO demonstrated widespread autonomic dysfunction, bladder dysfunction was observed in the seropositive patients with CIPO as a prominent clinical characteristic of dysautonomia.
Conclusions
These results demonstrate a significant prevalence of anti-gAChR antibodies in patients with achalasia and CIPO. Anti-gAChR Abs might mediate autonomic dysfunction, contributing to autoimmune mechanisms underlying these GI motility disorders.





Similar content being viewed by others
Abbreviations
- AAG:
-
Autoimmune autonomic ganglionopathy
- Abs:
-
Antibodies
- AGID:
-
Autoimmune gastrointestinal dysmotility
- AI:
-
Antibody index
- CIPO:
-
Chronic intestinal pseudo-obstruction
- COMPASS 31:
-
Composite autonomic symptom score 31
- DES:
-
Diffuse esophageal spasms
- gAChR:
-
Ganglionic acetylcholine receptor
- GAD:
-
Glutamic acid decarboxylase
- GI dysmotility:
-
Gastrointestinal dysmotility
- HC:
-
Healthy controls
- H/M:
-
Heart to mediastinum
- IVMP:
-
Intravenous methylprednisolone
- IVIg:
-
Intravenous immunoglobulin
- LIPS:
-
Luciferase immunoprecipitation system
- OH:
-
Orthostatic hypotension
- OI:
-
Orthostatic intolerance
- PLEX:
-
Plasma exchange
- PSL:
-
Prednisolone
- RLU:
-
Relative luminescence units
- VGCC:
-
Voltage-gated calcium channel
- VGKC:
-
Voltage-gated potassium channel
References
Dhamija R, Tan KM, Pittock SJ, et al. Serologic profiles aiding the diagnosis of autoimmune gastrointestinal dysmotility. Clin Gastroenterol Hepatol. 2008;6:988–92.
Flanagan EP, Saito YA, Lennon VA, et al. Immunotherapy trial as diagnostic test in evaluating patients with presumed autoimmune gastrointestinal dysmotility. Neurogastroenterol Motil. 2014;26:1285–97.
Verne GN, Sallustio JE, Eaker EY. Anti-myenteric neuronal antibodies in patients with achalasia. A prospective study. Dig Dis Sci. 1997;42:307–13.
Kraichely RE, Farrugia G, Pittock SJ, et al. Neural autoantibody profile of primary achalasia. Dig Dis Sci. 2010;55:307–11.
Hubball AW, Lang B, Souza MA, et al. Voltage-gated potassium channel (K(v) 1) autoantibodies in patients with chagasic gut dysmotility and distribution of K(v) 1 channels in human enteric neuromusculature (autoantibodies in GI dysmotility). Neurogastroenterol Motil. 2012;24:719–28.
Goin JC, Sterin-Borda L, Bilder CR, et al. Functional implications of circulating muscarinic cholinergic receptor autoantibodies in chagasic patients with achalasia. Gastroenterology. 1999;117:798–805.
Törnblom H, Lang B, Clover L, et al. Autoantibodies in patients with gut motility disorders and enteric neuropathy. Scand J Gastroenterol. 2007;42:1289–93.
Gyawall CP. Achalasia: new perspectives on an old disease. Neurogastroenterol Motil. 2016;28:4–11.
Booy JD, Takata J, Tomlinson G, et al. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis Esophagus. 2012;25:209–13.
O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19:5806–12.
Quidute AR, Freitas EV, Lima TG, et al. Achalasia and thyroid disease: possible autoimmune connection? Arq Bras Endocrinol Metabol. 2012;56:677–82.
Verne GN, Hahn AB, Pineau BC, et al. Association of HLA-DR and -DQ alleles with idiopathic achalasia. Gastroenterology. 1999;117:26–31.
Di Nardo G, Di Lorenzo C, Lauro A, et al. Chronic intestinal pseudo-obstruction in children and adults: diagnosis and therapeutic options. Neurogastroenterol Motil. 2017;29:e12945.
Bernardi MP, Warrier S, Lynch AC, et al. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85:709–14.
Lennon VA, Sas DF, Busk MF, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology. 1991;100:137–42.
Nojima Y, Mimura T, Hamasaki K, et al. Chronic intestinal pseudoobstruction associated with autoantibodies against proliferating cell nuclear antigen. Arthritis Rheum. 1996;39:877–9.
Mok MY, Wong RW, Lau CS. Intestinal pseudo-obstruction in systemic lupus erythematosus: an uncommon but important clinical manifestation. Lupus. 2000;9:11–8.
Viallard JF, Vincent A, Moreau JF, et al. Thymoma-associated neuromyotonia with antibodies against voltage-gated potassium channels presenting as chronic intestinal pseudo-obstruction. Eur Neurol. 2005;53:60–3.
Nunokawa T, Yokogawa N, Ohtsuka H, et al. Transgastric long tube placement following percutaneous endoscopic gastrostomy for severe chronic intestinal pseudo-obstruction related to systemic sclerosis. Mod Rheumatol. 2015;25:958–61.
Nakane S, Higuchi O, Koga M, et al. Clinical features of autoimmune autonomic ganglionopathy and the detection of subunit-specific autoantibodies to the ganglionic acetylcholine receptor in Japanese patients. PLoS ONE. 2015;10:e0118312.
Morimoto N, Takahashi S, Inaba T, et al. A case of seropositive autoimmune autonomic ganglionopathy with diffuse esophageal spasm. J Clin Neurosci. 2017;39:90–2.
Kawanishi K, Moribata K, Kato J, et al. A case report of chronic intestinal pseudo-obstruction with autoimmune autonomic ganglionopathy suspected from seropositive results for anti-ganglionic acetylcholine receptor antibody. Nihon Shokakibyo Gakkai Zasshi. 2015;112:62–9 [Japanese].
Vernino S, Low PA, Fealey RD, et al. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–55.
Nakane S, Mukaino A, Maeda Y, et al. Extra-autonomic manifestations in autoimmune autonomic ganglionopathy: a Japanese survey. J Neurol Neurosurg Psychiatr. 2017;88:367–8.
Ohkubo H, Iida H, Takahashi H, et al. An epidemiologic survey of chronic intestinal pseudo-obstruction and evaluation of the newly proposed diagnostic criteria. Digestion. 2012;86:12–9.
Iida H, Ohkubo H, Inamori M, et al. Epidemiology and clinical experience of chronic intestinal pseudo-obstruction in Japan: a nationwide epidemiologic survey. J Epidemiol. 2013;23:288–94.
Mukaino A, Nakane S, Higuchi O, et al. Insights from the ganglionic acetylcholine receptor autoantibodies in patients with Sjögren’s syndrome. Mod Rheumatol. 2016;26:708–15.
Maeda Y, Migita K, Higuchi O, et al. Association between anti-ganglionic nicotinic acetylcholine receptor (gAChR) antibodies and HLA-DRB1 alleles in the Japanese population. PLoS ONE. 2016;11:e0146048.
Maeda Y, Nakane S, Higuchi O, et al. Ganglionic acetylcholine receptor autoantibodies in patients with autoimmune diseases including primary biliary cirrhosis. Mod Rheumatol. 2017;27:664–8.
Sletten DM, Suarez GA, Low PA, et al. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87:1196–201.
Cortez MM, Reddy SKN, Goodman B, et al. Autonomic symptom burden is associated with MS-related fatigue and quality of life. Mult Scler Relat Disord. 2015;4:258–63.
Wang Z, Low PA, Jordan J, et al. Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current. Neurology. 2007;68:1917–21.
Vernino S, Lindstrom J, Hopkins S, et al. Characterization of ganglionic acetylcholine receptor autoantibodies. J Neuroimmunol. 2008;197:63–9.
Wang Z, Low PA, Vernino S. Antibody-mediated impairment and homeostatic plasticity of autonomic ganglionic synaptic transmission. Exp Neurol. 2010;222:114–9.
Drachman DB. Autonomic “myasthenia”: the case for an autoimmune pathogenesis. J Clin Invest. 2003;111:797–9.
Minami H, Isomoto H, Yamaguchi N, et al. Peroral endoscopic myotomy for esophageal achalasia: clinical impact of 28 cases. Dig Endosc. 2014;26:43–51.
McMillan HJ, Srinivasan J. Achalasia, chronic sensory neuropathy, and N-type calcium channel autoantibodies: beneficial response to IVIG. Clin J Gastroenterol. 2010;3:78–82.
Acknowledgements
This study was supported by the Neuroimmunological Disease Research Committee and the Ministry of Health, Labor, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (JSPS KAKENHI Grant Number 25461305).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: AM, HM (Hitomi Minami), HI, OH, HM (Hidenori Matsuo), KN, SN. Performed the experiments: AM, YM, OH. Collected the samples and summarized the cases: AM, HM (Hitomi Minami), HI, HH, EI, TO, YK, KD, FS, TU, KM, TY, KM, YO, AI, KN. Analyzed the data: AM, HM (Hitomi Minami), HI, HH, OH, SN. Wrote the paper: AM, HM (Hitomi Minami), HI, OH, AI, HM (Hidenori Matsuo), KN, SN.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mukaino, A., Minami, H., Isomoto, H. et al. Anti-ganglionic AChR antibodies in Japanese patients with motility disorders. J Gastroenterol 53, 1227–1240 (2018). https://doi.org/10.1007/s00535-018-1477-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1477-8