Abstract
Background
Dendritic cells (DCs), primary antigen-presenting cells, are now well known as an immunoregulator of many aspects of immune responses including inflammatory bowel diseases (IBDs) such as Crohn’s disease and ulcerative colitis. We have reported that PIR-A/Bhigh cDCs (conventional DCs) appeared in dextran sodium sulfate (DSS)-induced colitis and serve as a negative immunoregulator in an animal model of IBD. The immunoregulatory role of PIR-A/B+ cDCs was confirmed in both an in vitro culture system and an in vivo transfer experiment. Here, we have investigated the differentiation process of PIR-A/B+ cDCs in an in vitro inflammatory environment and examined their functions.
Methods
cDCs were isolated from the large intestinal lamina propria from C57BL/6 mice and cultured in an inflammatory environment (IL-1, IL-6, TNFα, and LPS). The appearance of PIR-A/B+ cDCs was determined after 24 h, and the in vitro-induced PIR-A/B+ cDCs were functionally and genetically examined.
Results
PIR-A/B+ cDCs were detected after a 24-h culture only in the inflammatory environment, and the cells acted as a negative immunoregulator when examined in an allogenic mixed leukocyte reaction (MLR). The message level of IL-27 was highly upregulated in PIR-A/B+ cDCs, while that of high mobility group box 1 protein (HMGB1) was downregulated in these cells. This was well in accordance with the fact that PIR-A/B+ cDCs showed a suppressive function against activated T cells. We found that PIR-A/B+ cDCs produced IL-27, as verified by an ELISA assay, and that the inhibitory effect by PIR-A/B+ cDCs was, at least partially, due to IL-27. Furthermore, CD85d+ cells, a human counterpart of mouse PIR-A/B+ cDCs, were found in the lamina propria of the colon of the patients with ulcerative colitis, but not in the similar part of the non-inflammatory area of colon specimens from patients with colon cancer.
Conclusions
PIR-A/B+ cDCs induced in an in vitro inflammatory environment model showed a suppressive function against activated T cells by producing an inhibitory cytokine.







Similar content being viewed by others

References
Steinman RM, Hawiger D, Liu K, et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25.
Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–56.
Kretschmer K, Apostlou I, Hawiger D, et al. Inducing and expanding regulatory T cell population by foreign antigen. Nat Immunol. 2005;6:1219–27.
Steinman RM, Turley S, Mellman I, et al. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–6.
Molleri AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46.
Wakkach A, Fournier N, Brun V, et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17.
Chorny A, Gonzalez-Rey E, Fernandez-Martin A, et al. Vasoactive intestinal peptide induce regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood. 2006;107:3787–94.
Sato K, Yamashita N, Baba M, et al. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–79.
Sato K, Yamashita N, Baba M, et al. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003;101:3581–9.
Fujita S, Sato Y, Sato K, et al. Regulatory dendritic cells protect against cutaneous chronic graft-versus-host disease mediated through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2007;110:3793–803.
Hoshino S, Inaba M, Iwai H, et al. The role of dendritic cell subsets in 2,4,6-trinitrobenzene sulfonic acid-induced ileitis. J Autoimmun. 2010;34:380–9.
Hoshino S, Kurishima A, Inaba M, et al. Amelioration of 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice by immunoregulatory dendritic cells. J Gastroenterol. 2011;46:1368–81.
Kurishima A, Inaba M, Sakaguchi Y, et al. Immunoregulatory function of PIR-A/B+ DCs in the inflammatory response of dextran sodium sulfate-induced colitis. J Gastoenterol. 2014;49:1367–77.
Berndt BE, Zhang M, Chen GH, et al. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol. 2007;179:6255–62.
Danese S. Nonimmune cells in inflammatory bowel disease: from victim to villain. Trends Immunol. 2008;29:555–64.
Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest. 2009;119:2441–50.
Abe K, Nguyen KP, Fine SD, et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci USA. 2007;104:17022–7.
Fahlén L, Read S, Gorelik L, et al. T cells that cannot respond to TGF-β escape control by CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201:737–46.
Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+ CD25+ T cells. J Autoimmun. 2001;16:115–23.
Groux H, O’Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42.
Bilsborough J, George TC, Norment A, et al. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–92.
Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604.
Villarino AV, Hunter CA. Biology of recently discovered cytokines: discerning the pro- and anti-inflammatory properties of interleukin-27. Arthritis Res Ther. 2004;6:225–33.
Chen Y, Jiang G, Yang H-R, et al. Distinct response of liver myeloid dendritic cells to endotoxin is mediated by IL-27. J Hepatol. 2009;51:510–9.
Dann SM, Le C, Choudhury BK, et al. Attenuation of intestinal inflammation in interleukin-10-deficient mice infected with Citrobacter rodentium. Infect Immun. 2014;81:1949–58.
Mora JR, Bono MR, Manjunath N, Weninger W, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93.
Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40:1877–83.
Siddiqui KR, Laffont S, Poerie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–67.
Mascarell L, Airouche S, Berjont N, et al. The regulatory dendritic cell marker C1q is a potent inhibitor of allergic inflammation. Mucosal Immunol. 2017;10(3):695–704. https://doi.org/10.1038/mi.2016.87 (Epub 2016 Oct 12).
Uto T, Fukaya T, Takagi H, et al. Clec4A4 is a regulatory receptor for dendritic cells that impairs inflammation and T-cell immunity. Nat Commun. 2016;12(7):11273. https://doi.org/10.1038/ncomms11273.
Acknowledgements
The authors thank Dr. Hiroyuki Gonda, Central Research of Laboratory, for his help with the flow cytometry analyses. We also thank Mari Mino-Kendson MD., Dept. Pathology, Massachusetts General Hospital, for her helpful advice in pathological studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2018_1447_MOESM1_ESM.jpg
Supplementary Figure 1. Appearance of PIR-A/B+ cDC subset in mice with DSS-induced colitis. The colon-derived DC-enriched population was stained with FITC–anti-CD11c, PE–anti-PIR-A/B, and APC–anti-CD45R mAbs, and the cells with the PIR-A/B+ immunophenotypes in the B220- (CD11c+) DCs were determined on days 0 (before the induction of colitis, A) and 5 after the treatment of DSS (B). The result shown here is representative of three replicate experiments. Dead cells were gated out by stain with propidium iodide. (JPEG 333 kb)
535_2018_1447_MOESM2_ESM.jpg
Supplementary Figure 2. Effect of IFNα in the induction of PIR-A/B+ cDCs. The colon-derived cDCs were prepared from normal mice, and PIR-A/B- cDCs were sorted using a FACSAria. The sorted PIR-A/B- cDCs were cultured with [GM-CSF (1μg/m/l) + IL-1 (10 ng/ml) + IL-6 (10 ng/ml) + TNFα (10 ng/ml)] (A), [GM-CSF + IL-1+ IL-6 + TNFα + IFNα (10 ng/ml)] (B), or [GM-CSF + IL-1 + IL-6 + TNFα + LPS (100 ng/ml)] (C). The DCs were harvested 48 hours later and the frequency of PIR-A/B+ cDCs (shown as gate R3 in the dot plot) were assessed using a FACS Calibur HG™ after staining with DCs with FITC–anti-CD11c mAb and PE–anti-PIR-A/B mAb. (JPEG 368 kb)
535_2018_1447_MOESM3_ESM.jpg
Supplementary Figure 3. Heat map and scatter plot. A heat map of cytokine-related genes is shown in A, and a scatter plot of PIR-A/B+ cDCs versus PIR-A/B- cDCs is shown in B. (JPEG 354 kb)
535_2018_1447_MOESM4_ESM.jpg
Supplementary Figure 3. Heat map and scatter plot. A heat map of cytokine-related genes is shown in A, and a scatter plot of PIR-A/B+ cDCs versus PIR-A/B- cDCs is shown in B. (JPEG 654 kb)
535_2018_1447_MOESM5_ESM.jpg
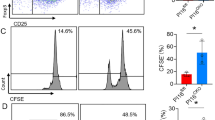
Supplementary Figure 4. Assessment of the inhibitory function of purified PIR-A/B+ cDCs by CFSE assay. The inhibitory function of purified PIR-A/B+ cDCs was examined by CFSE assay. Purified CD4+ T cells were stained with a CFSE reagent before the MLR culture. Four days later, the proliferative activity of CD4+ T cells was assessed using a FACSCanto II. In the histogram, the region M1 shows highly proliferative cells, and M2 shows moderately proliferative cells. The number in the figure shows the percentage of cells in each region (means and standard deviations of triplicated cultures. $, **, ##: p<0.01, #; p<0.05). It is noted that the stimulatory activity of PIR-A/B+ cDCs (E) is lower than that of splenic DCs (B). (JPEG 513 kb)
535_2018_1447_MOESM6_ESM.jpg
Supplementary Figure 5. Effect of anti-TGF-β Ab on the inhibitory function of PIR-A/B+ cDCs. Effect of anti-TGF-β Ab on the inhibitory function of PIR-A/B+ cDCs was examined in MLR and by CFSE assay. Purified CD4+ T cells were stimulated with splenic DCs (B), and in vitro-generated PIR-A/B+ cDCs were added to MLR (C). Furthermore, anti-IL-27 Ab or anti-TGF-β Ab was added to MLR to assess the effect on on the inhibitory function of PIR-A/B+ cDCs. The number in the figure shows the percentage of cells in each region (means and standard deviations of triplicated cultures. $; p<0.01, **; p<0.05). (JPEG 502 kb)
535_2018_1447_MOESM7_ESM.jpg
Supplementary Figure 6. IL-2 production by CD4+ T cells in MLR. IL-2 in the supernatant of the MLR was determined using an ELISA kit for IL-2. Columns and bars in the figure represent means and standard deviations of triplicated cultures. (JPEG 398 kb)
535_2018_1447_MOESM8_ESM.xls
Footnote for Supplementary Table 1. The expression of cytokine-related genes assayed by a microarray analysis is represented as excel data. (XLS 1444 kb)
Rights and permissions
About this article
Cite this article
Matsui, F., Inaba, M., Uchida, K. et al. Induction of PIR-A/B+ DCs in the in vitro inflammatory condition and their immunoregulatory function. J Gastroenterol 53, 1131–1141 (2018). https://doi.org/10.1007/s00535-018-1447-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1447-1