Abstract
Background
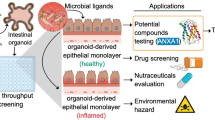
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon with an intractable, recurrent course. The goal of UC therapy is to target mucosal healing because immune-suppressive therapy for UC frequently results in relapse. However, few drugs directly target mucosal healing. We, therefore, aim to evaluate the therapeutic effect of an investigational drug on intestinal epithelial cells in an in vitro UC model using human colonic organoids.
Methods
Colonic organoids were isolated from human colon and cultured. A mixture of cytokines and bacterial components were used to mimic UC in humans. The effect of the investigational drug on colonic organoid was evaluated by microarray analysis and 3D immunofluorescence. The enrichment of stem cells was assessed with a colony formation assay.
Results
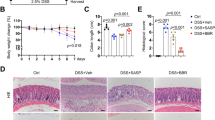
Inflammatory stimulation resulted in a significant induction of inflammatory-related genes in colonic organoids whereas cell differentiation was suppressed. Treatment with the investigational drug KAG-308 showed reciprocal dynamics of gene expression to inflammatory stimulation, which resulted in not only the suppression of immune response but also the promotion of cellular differentiation towards secretory lineages. Moreover, SPDEF and Reg4 were identified as novel targets for the enrichment of intestinal epithelial stem cells and mucosal healing.
Conclusions
The establishment of in vitro UC model using human colonic organoid could reveal the effects and targets of investigational drugs in intestinal epithelial cells under inflammation conditions. Further maturation of this system might be more efficient to predict the effect on UC, as compared with the use of animal model, for the development of new drugs.





Similar content being viewed by others
Abbreviations
- IBD:
-
Inflammatory bowel disease
- UC:
-
Ulcerative colitis
- DSS:
-
Dextran sulphate sodium
- AOM:
-
Azoxymethane
- IECs:
-
Intestinal epithelial cells
- NF-κB:
-
Nuclear factor-κB
- PGE2:
-
Prostaglandin E2
- EP4:
-
Prostaglandin E2 receptor 4
- GSEA:
-
Gene set enrichment analysis
- TLRs:
-
Toll-like receptors
- IL:
-
Interleukin
- ROS:
-
Reactive oxygen species
- LPS:
-
Lipopolysaccharide
- TNF:
-
Tumour necrosis factor
- CLDN18:
-
Claudin-18
- CIITA:
-
Class II major histocompatibility complex transactivator
- DAPI:
-
4′,6-diamidino-2-phenylindole
- Reg4:
-
Regenerating family member 4
- SPDEF:
-
SAM-pointed domain containing ETS transcription factor
- Duoxa2:
-
Dual oxidase maturation factor 2
- ALDH1:
-
Aldehyde dehydrogenase 1
- Lgr5:
-
Leucine-rich repeat-containing G-protein coupled receptor 5
- Hes1:
-
Hairy and enhancer of split 1
- Atoh1:
-
Atonal bHLH transcription factor 1
- TFF3:
-
Trefoil factor 3
- MUC2:
-
Mucin 2
- CgA:
-
Chromogranin A
References
Arora Z, Shen B. Biological therapy for ulcerative colitis. Gastroenterol Rep. 2015;3:103–9.
Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380:1606–19.
Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19.
Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769–84.
Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016;51:11–21.
Valatas V, Vakas M, Kolios G. The value of experimental models of colitis in predicting efficacy of biological therapies for inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2013;305:G763–85.
Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–23.
Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Hibiya S, Tsuchiya K, Hayashi R, et al. Long-term inflammation transforms intestinal epithelial cells of colonic organoids. J Crohns Colitis. 2017;11:621–30.
MacFie TS, Poulsom R, Parker A, et al. DUOX2 and DUOXA2 form the predominant enzyme system capable of producing the reactive oxygen species H2O2 in active ulcerative colitis and are modulated by 5-aminosalicylic acid. Inflamm Bowel Dis. 2014;20:514–24.
Miyoshi H, VanDussen KL, Malvin NP, et al. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J. 2017;36:5–24.
Kawahara K, Hohjoh H, Inazumi T, et al. Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta. 2015;1851:414–21.
O’Callaghan G, Houston A. Prostaglandin E2 and the EP receptors in malignancy: possible therapeutic targets? Br J Pharmacol. 2015;172:5239–50.
Watanabe Y, Murata T, Amakawa M, et al. KAG-308, a newly-identified EP4-selective agonist shows efficacy for treating ulcerative colitis and can bring about lower risk of colorectal carcinogenesis by oral administration. Eur J Pharmacol. 2015;754:179–89.
Fujii S, Suzuki K, Kawamoto A, et al. PGE2 is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells. Sci Rep. 2016;6:36795.
Planell N, Lozano JJ, Mora-Buch R, et al. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut. 2013;62:967–76.
Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut. 1999;44:890–5.
Zwiers A, Fuss IJ, Leijen S, et al. Increased expression of the tight junction molecule claudin-18 A1 in both experimental colitis and ulcerative colitis. Inflamm Bowel Dis. 2008;14:1652–9.
Dotti I, Mora-Buch R, Ferrer-Picon E, et al. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut. 2017;66:2069–79.
Kazanjian A, Noah T, Brown D, et al. Atonal homolog 1 is required for growth and differentiation effects of notch/gamma-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 2010;139(918–928):928.e911–6.
Zheng X, Tsuchiya K, Okamoto R, et al. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm Bowel Dis. 2011;17:2251–60.
Lo YH, Chung E, Li Z, et al. Transcriptional regulation by ATOH1 and its target SPDEF in the intestine. Cell Mol Gastroenterol Hepatol. 2017;3:51–71.
Sasaki N, Sachs N, Wiebrands K, et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci USA. 2016;113:E5399–407.
Nanakin A, Fukui H, Fujii S, et al. Expression of the REG IV gene in ulcerative colitis. Lab Investig. 2007;87:304–14.
Jiang GL, Nieves A, Im WB, et al. The prevention of colitis by E Prostanoid receptor 4 agonist through enhancement of epithelium survival and regeneration. J Pharmacol Exp Ther. 2007;320:22–8.
Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15:196–212.
Xia K, Xue H, Dong D, et al. Identification of the proliferation/differentiation switch in the cellular network of multicellular organisms. PLoS Comput Biol. 2006;2:e145.
Tsuchiya K, Nakamura T, Okamoto R, et al. Reciprocal targeting of Hath1 and beta-catenin by wnt glycogen synthase kinase 3 beta in human colon cancer. Gastroenterology. 2007;132:208–20.
Okamoto R, Tsuchiya K, Nemoto Y, et al. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G23–35.
Izumi H, Minegishi M, Sato Y, et al. Bifidobacterium breve alters immune function and ameliorates DSS-induced inflammation in weanling rats. Pediatr Res. 2015;78:407–16.
Acknowledgements
This work was supported by scientific Research, from the Japanese Ministry of Education, Culture, Sports, Science and Technology as follows (KAKENHI grant numbers 24590935, 25114703, 25130704, 26221307, 15H04808, 16H06770, 17H06654, 17K15930, 17K19513); Japan Foundation for Applied Enzymology; the Health and Labor Sciences Research Grant from the Japanese Ministry of Health, Labor and Welfare (MHLW) as follows (Grant number 14526073); The Practical Research for Innovative Cancer Control, Rare/Intractable Diseases from Japan Agency for Medical Research and Development (AMED) as follows (Grant numbers 15Ack0106017h0002, 16ck0106017h0003, 16ek0109456h0003); Research Grant of the Princess Takamatsu Cancer Research Fund; Naoki Tsuchida Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YM and YW are employed by Kaken Pharmaceutical Co., Ltd. KT has received research funding (collaborative research) provided by Kaken Pharmaceutical Co., Ltd.
Author contributions
MW, TN and KT designed the experiments and acquired data. TS and SH established inflammatory stimulation of the organoids. RN and SW assessed the effect of KAG-308 on the organoids. YM and YW assessed the effect of KAG-308 on DSS colitis model in mice. KT and MW coordinated the projects and drafted the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nishimura, R., Shirasaki, T., Tsuchiya, K. et al. Establishment of a system to evaluate the therapeutic effect and the dynamics of an investigational drug on ulcerative colitis using human colonic organoids. J Gastroenterol 54, 608–620 (2019). https://doi.org/10.1007/s00535-018-01540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-01540-y