Abstract
Background
Congenital biliary dilatation (CBD) is a congenital malformation involving both dilatation of the extrahepatic bile duct and pancreaticobiliary maljunction. Persistent reflux of pancreatic juice injures the biliary tract mucosa, resulting in chronic inflammation and higher rates of carcinogenesis in the biliary tract, including the gallbladder. Telomeres are repetitive DNA sequences located at the ends of chromosomes. Chromosomal instability due to telomere dysfunction plays an important role in the carcinogenesis of many organs. This study was performed to determine whether excessive shortening of telomeres occurs in the gallbladder mucosa of patients with CBD.
Methods
Resected gallbladders were obtained from 17 patients with CBD, ten patients with cholecystolithiasis without pancreatic juice reflux, and 17 patients with normal gallbladders (controls) (median age of each group of patients: 37, 50, and 53 years, respectively). The telomere lengths of the gallbladder epithelium were measured by quantitative fluorescence in situ hybridization using tissue sections, and the normalized telomere-to-centromere ratio (NTCR) was calculated.
Results
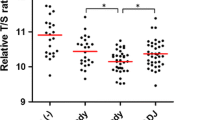
The NTCRs in the CBD, cholecystolithiasis, and control groups were 1.24 [interquartile range (IQR) 1.125–1.52], 1.96 (IQR 1.56–2.295), and 1.77 (IQR 1.48–2.53), respectively. The NTCR in the CBD group was significantly smaller than that in the cholecystolithiasis and control groups (p = 0.003 and 0.004, respectively), even in young patients.
Conclusions
Our findings indicate that telomere shortening in the gallbladder mucosa plays an important role in the process of carcinogenesis in patients with CBD. These results support the recommendation of established guidelines for prophylactic surgery in patients with CBD because CBD is a premalignant condition with excessive telomere shortening.





Similar content being viewed by others
Change history
05 December 2017
In the original publication of this article, Fig. 5 was published with incorrect color of the approximate lines of CBD and Control.
References
Hamada Y, Ando H, Kamisawa T, et al. Diagnostic criteria for congenital biliary dilatation 2015. J Hepatobiliary Pancreat Sci. 2016;23:342–6.
Ishibashi H, Shimada M, Kamisawa T, et al. Japanese clinical practice guidelines for congenital biliary dilatation. J Hepatobiliary Pancreat Sci. 2017;24:1–16.
Kamisawa T, Ando H, Hamada Y, et al. Diagnostic criteria for pancreaticobiliary maljunction 2013. J Hepatobiliary Pancreat Sci. 2014;21:159–61.
Kamisawa T, Takuma K, Anjiki H, et al. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol. 2009;7:S84–8.
Kamisawa T, Egawa N, Nakajima H, et al. Origin of the long common channel based on pancreatographic findings in pancreaticobiliary maljunction. Dig Liver Dis. 2005;37:363–7.
Kamisawa T, Kuruma S, Tabata T, et al. Pancreaticobiliary maljunction and biliary cancer. J Gastroenterol. 2015;50:273–9.
Fujimoto T, Ohtsuka T, Nakashima Y, et al. Elevated bile amylase level without pancreaticobiliary maljunction is a risk factor for gallbladder carcinoma. J Hepatobiliary Pancreat Sci. 2017;24:103–8.
Li Y, Wei J, Zhao Z, et al. Pancreaticobiliary maljunction is associated with common bile duct carcinoma: a meta-analysis. Sci World J. 2013;2013:618670.
Deng YL, Cheng NS, Lin YX, et al. Relationship between pancreaticobiliary maljunction and gallbladder carcinoma: meta-analysis. Hepatobiliary Pancreat Dis Int. 2011;10:570–80.
Morine Y, Shimada M, Takamatsu H, et al. Clinical features of pancreaticobiliary maljunction: update analysis of 2nd Japan-nationwide survey. J Hepatobiliary Pancreat Sci. 2013;20:472–80.
Matsuda T, Marugame T, Kamo K, et al. Cancer incidence and incidence rates in Japan in 2003: based on data from 13 population-based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn J Clin Oncol. 2009;39:850–8.
Kamisawa T, Ando H, Suyama M, et al. Japanese clinical practice guidelines for pancreaticobiliary maljunction. J Gastroenterol. 2012;47:731–59.
Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6.
Sandell LL, Zakian VA. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–39.
Blackburn EH. Telomeres: no end in sight. Cell. 1994;77:621–3.
Takubo K, Izumiyama-Shimomura N, Honma N, et al. Telomere lengths are characteristic in each human individual. Exp Gerontol. 2002;37:523–31.
Ishikawa N, Nakamura K, Izumiyama-Shimomura N, et al. Changes of telomere status with aging: an update. Geriatr Gerontol Int. 2016;16(1):30–42.
Takubo K, Nakamura K, Izumiyama N, et al. Telomere shortening with aging in human liver. J Gerontol A Biol Sci Med Sci. 2000;55:B533–6.
O’Sullivan JN, Bronner MP, Brentnall TA, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280–4.
Saretzki G, Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci. 2002;959:24–9.
Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–5.
Aida J, Yokoyama A, Izumiyama N, et al. Alcoholics show reduced telomere length in the oesophagus. J Pathol. 2011;223:410–6.
DePinho RA. The age of cancer. Nature. 2000;408:248–54.
Takubo K, Aida J, Izumiyama-Shimomura N, et al. Changes of telomere length with aging. Geriatr Gerontol Int. 2010;10(1):S197–206.
Takubo K, Nakamura K, Izumiyama N, et al. Telomere shortening with aging in human esophageal mucosa. Age (Omaha). 1999;22:95–9.
Lansdorp PM, Verwoerd NP, van de Rijke FM, et al. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–91.
Meeker AK, Gage WR, Hicks JL, et al. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259–68.
Ferlicot S, Youssef N, Feneux D, et al. Measurement of telomere length on tissue sections using quantitative fluorescence in situ hybridization (Q-FISH). J Pathol. 2003;200:661–6.
Kammori M, Onoda N, Nakamura K, et al. Specific subtelomere loss on chromosome der (11) t (3; 11) (q23; q23) × 2 in anaplastic thyroid cancer cell line OCUT-1. Int J Mol Med. 2006;18:9–16.
Aida J, Izumiyama-Shimomura N, Nakamura K, et al. Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol. 2007;38:1192–200.
Kammori M, Poon SS, Nakamura K, et al. Squamous cell carcinomas of the esophagus arise from a telomere-shortened epithelial field. Int J Mol Med. 2007;20:793–9.
Aida J, Izumiyama-Shimomura N, Nakamura K, et al. Basal cells have longest telomeres measured by tissue Q-FISH method in lingual epithelium. Exp Gerontol. 2008;43:833–9.
Kurabayashi R, Takubo K, Aida J, et al. Luminal and cancer cells in the breast show more rapid telomere shortening than myoepithelial cells and fibroblasts. Hum Pathol. 2008;39:1647–55.
Shiraishi H, Mikami T, Aida J, et al. Telomere shortening in Barrett’s mucosa and esophageal adenocarcinoma and its association with loss of heterozygosity. Scand J Gastroenterol. 2009;44:538–44.
Aida J, Izumo T, Shimomura N, et al. Telomere lengths in the oral epithelia with and without carcinoma. Eur J Cancer. 2010;46:430–8.
Takubo K, Aida J, Izumiyama N, et al. Chromosomal instability and telomere lengths of each chromosomal arm measured by Q-FISH in human fibroblast strains prior to replicative senescence. Mech Ageing Dev. 2010;131:614–24.
Takubo K, Fujita M, Izumiyama N, et al. Q-FISH analysis of telomere and chromosome instability in the oesophagus with and without squamous cell carcinoma in situ. J Pathol. 2010;221:201–9.
Sanada Y, Aida J, Kawano Y, et al. Hepatocellular telomere length in biliary atresia measured by Q-FISH. World J Surg. 2012;36:908–16.
Kawano Y, Ishikawa N, Aida J, et al. Q-FISH measurement of hepatocyte telomere lengths in donor liver and graft after pediatric living-donor liver transplantation: donor age affects telomere length sustainability. PLoS One. 2014;9:e93749.
Todani T, Watanabe Y, Narusue M, et al. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–9.
Ohashi M, Aizawa S, Ooka H, et al. A new human diploid cell strain, TIG-1, for the research on cellular aging. Exp Gerontol. 1980;15:121–33.
Funabiki T, Matsubara T, Miyakawa S, et al. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;394:159–69.
Seki M, Yanagisawa A, Ninomiya E, et al. Clinicopathology of pancreaticobiliary maljunction: relationship between alterations in background biliary epithelium and neoplastic development. J Hepatobiliary Pancreat Surg. 2005;12:254–62.
Tsuchida A, Itoi T, Aoki T, et al. Carcinogenetic process in gallbladder mucosa with pancreaticobiliary maljunction (Review). Oncol Rep. 2003;10:1693–9.
Matsumoto Y, Fujii H, Itakura J, et al. Pancreaticobiliary maljunction: pathophysiological and clinical aspects and the impact on biliary carcinogenesis. Langenbecks Arch Surg. 2003;388:122–31.
Shimada K, Yanagisawa J, Nakayama F. Increased lysophosphatidylcholine and pancreatic enzyme content in bile of patients with anomalous pancreaticobiliary ductal junction. Hepatology. 1991;13:438–44.
Tsuchida A, Itoi T. Carcinogenesis and chemoprevention of biliary tract cancer in pancreaticobiliary maljunction. World J Gastrointest Oncol. 2010;2:130–5.
Kamisawa T, Kuruma S, Chiba K, et al. Biliary carcinogenesis in pancreaticobiliary maljunction. J Gastroenterol. 2017;52:158–63.
Kamisawa T, Funata N, Hayashi Y, et al. Pathologic changes in the non-carcinomatous epithelium of the gallbladder in patients with a relatively long common channel. Gastrointest Endosc. 2004;60:56–60.
Sai JK, Suyama M, Nobukawa B, et al. Precancerous mucosal changes in the gallbladder of patients with occult pancreatobiliary reflux. Gastrointest Endosc. 2005;61:264–8.
Beltran MA, Vracko J, Cumsille MA, et al. Occult pancreaticobiliary reflux in gallbladder cancer and benign gallbladder diseases. J Surg Oncol. 2007;96:26–31.
Horaguchi J, Fujita N, Noda Y, et al. Amylase levels in bile in patients with a morphologically normal pancreaticobiliary ductal arrangement. J Gastroenterol. 2008;43:305–11.
Kamisawa T, Suyama M, Fujita N, et al. Pancreatobiliary reflux and the length of a common channel. J Hepatobiliary Pancreat Sci. 2010;17:865–70.
Matsuda M, Watanabe G, Hashimoto M, et al. Evaluation of pancreaticobiliary maljunction and low bile amylase levels (in Japanese with English abstract). Tando J Jpn Biliary Assoc. 2007;21:119–24.
Matsuda Y, Ishiwata T, Izumiyama-Shimomura N, et al. Gradual telomere shortening and increasing chromosomal instability among PanIN grades and normal ductal epithelia with and without cancer in the pancreas. PLoS One. 2015;10:e0117575.
Izumiyama-Shimomura N, Nakamura K, Aida J, et al. Short telomeres and chromosome instability prior to histologic malignant progression and cytogenetic aneuploidy in papillary urothelial neoplasms. Urol Oncol. 2014;32:135–45.
Ikeda H, Aida J, Hatamochi A, et al. Quantitative fluorescence in situ hybridization measurement of telomere length in skin with/without sun exposure or actinic keratosis. Hum Pathol. 2014;45:473–80.
Aida J, Yokoyama A, Shimomura N, et al. Telomere shortening in the esophagus of Japanese alcoholics: relationships with chromoendoscopic findings, ALDH2 and ADH1B genotypes and smoking history. PLoS One. 2013;8:e63860.
Aida J, Kobayashi T, Saku T, et al. Short telomeres in an oral precancerous lesion: Q-FISH analysis of leukoplakia. J Oral Pathol Med. 2012;41:372–8.
Aida S, Aida J, Hasegawa K, et al. Telomere length of human adult bronchial epithelium and bronchogenic squamous cell carcinoma measured using tissue quantitative fluorescence in situ hybridization. Respiration. 2015;90:321–6.
Research Team for Geriatric Pathology TMIoG. A proposed new definition for the concept of precancerous conditions. 2015. http://www.ttaggg-rtgp.org/assets/files/document/150413E.pdf. Accessed 02 Aug 2017.
Poojary SS, Mishra G, Gupta S, et al. Dysfunction of subtelomeric methylation and telomere length in gallstone disease and gallbladder cancer patients of North Central India. J Hepatobiliary Pancreat Sci. 2016;23:276–82.
Acknowledgements
We would like to thank Prof. Akira Shimizu of the Department of Analytic Human Pathology, Nippon Medical School, who cooperated in providing the specimens. We also thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s00535-017-1418-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aoki, Y., Aida, J., Kawano, Y. et al. Telomere length of gallbladder epithelium is shortened in patients with congenital biliary dilatation: measurement by quantitative fluorescence in situ hybridization. J Gastroenterol 53, 291–301 (2018). https://doi.org/10.1007/s00535-017-1411-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1411-5