Abstract
Background
Although tolvaptan is an effective treatment for hepatic edema, there are no established criteria for assessment of the therapeutic effect. The present study evaluates the association between body weight change and clinical symptoms to identify an effective indicator of tolvaptan response.
Methods
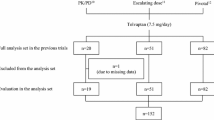
The study comprised 460 patients. The first data set contained 147 patients with hepatic edema who received tolvaptan in Kagoshima Kouseiren Hospital, a representative institution of this study. From these data, an optimal cutoff value of body weight change, which accurately indicated symptom reduction, was identified. The response rates obtained based on the cutoff value were evaluated by receiver-operating characteristic (ROC) analysis and kappa coefficients. The kappa coefficient was then validated internally using the bootstrap method and externally using the validation data set of 313 patients from four other hospitals.
Results
A cutoff value for body weight loss of 1.5 kg/week produced the largest area under the ROC curve (0.961; sensitivity, 89.8%; specificity, 92.0%) and a high kappa coefficient (0.831). The correlation between symptom reduction and body weight loss of 1.5 kg/week was evaluated internally and externally, and the cutoff value was validated.
Conclusions
The cutoff value of body weight change that most accurately reflected symptom reduction was 1.5 kg/week; this value is expected to be an effective indicator of response to tolvaptan in clinical practice.

Similar content being viewed by others
Abbreviations
- ROC:
-
Receiver-operating characteristic
- AQP2:
-
Aquaporin 2
- AUC:
-
Area under the ROC curve
- ASI-7:
-
Ascites Symptom Inventory-7
- CLDQ:
-
Chronic Liver Disease Questionnaire
References
Matsuzaki M, Hori M, Izumi T, Tolvaptan Investigators, et al. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther. 2011;25:33–45.
Okita K, Sakaida I, Okada M, et al. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–87.
Jujo K, Saito K, Ishida I, et al. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail. 2016;3:177–88.
Matsue Y, Suzuki M, Torii S, et al. Prognostic impact of early treatment with tolvaptan in patients with acute heart failure and renal dysfunction. Int J Cardiol. 2016;221:188–93.
Hanatani A, Shibata A, Kitada R, et al. Administration of tolvaptan with reduction of loop diuretics ameliorates congestion with improving renal dysfunction in patients with congestive heart failure and renal dysfunction. Heart Vessels. 2016;32:287–94 (Epub ahead of print).
Sakaida I, Kawazoe S, Kajimura K, et al. Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82.
Sakaida I, Yamashita S, Kobayashi T, et al. Efficacy and safety of a14-day administration of tolvaptan in the treatment of patients with ascites in hepatic oedema. J Int Med Res. 2013;41:835–47.
Hiramine Y, Uto H. Imamura Y, al. Efficacy of vasopressin V2 receptor antagonist tolvaptan in treatment of hepatic edema. Hepatol Res. 2016;. doi:10.1111/hepr.12778 (Epub ahead of print).
Uojima H, Kinbara T, Hidaka H, et al. Close correlation between urinary sodium excretion and response to tolvaptan in liver cirrhosis patients with ascites. Hepatol Res. 2016;. doi:10.1111/hepr.12716 (Epub ahead of print).
Sakaida I, Terai S, Nakajima K, et al. Predictive factors of the pharmacological action of tolvaptan in patients with liver cirrhosis: a post hoc analysis. J Gastroenterol. 2017;52:229–36.
Imamura T, Kinugawa K, Fujino T, et al. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J. 2014;78:2240–9.
Sakaida I, Nakajima K, Okita K, et al. Can serum albumin level affect the pharmacological action of tolvaptan in patients with liver cirrhosis? A post hoc analysis of previous clinical trials in Japan. J Gastroenterol. 2015;50:1047–53.
Yan L, Xie F, Lu J, et al. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2015;15:65.
Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–92.
Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55:1–12.
European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98–104.
Wong F, Watson H, Gerbes A, Satavaptan Investigators Group, et al. Satavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severity. Gut. 2012;61:108–16.
Izumi Y, Miura K, Iwao H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J Pharmacol Sci. 2014;124:1–6.
Imamura T, Kinugawa K, Shiga T, et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients-association between non-responders and chronic kidney disease. Circ J. 2013;77:397–404.
Iwamoto T, Maeda M, Hisanaga T, et al. Predictors of the effect of tolvaptan on the prognosis of cirrhosis. Intern Med. 2016;55:2911–6.
Kawaratani H, Fukui H, Moriya K, et al. Predictive parameter of tolvaptan effectiveness in cirrhotic ascites. Hepatol Res. 2016;. doi:10.1111/hepr.12826 (Epub ahead of print).
Nakanishi H, Kurosaki M, Hosokawa T, et al. Urinary excretion of the water channel aquaporin 2 correlated with the pharmacological effect of tolvaptan in cirrhotic patients with ascites. J Gastroenterol. 2016;51:620–7.
Zhang X, Wang SZ, Zheng JF, et al. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol. 2014;20:11400–5.
Imamura T, Kinugawa K, Ohtani T, et al. Assessment of quality of life during long-term treatment of tolvaptan in refractory heart failure: design and rationale of the AQUA-TLV study. Int Heart J. 2014;55:264–7.
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18.
O’Brien A, China L, Gant V. The potential danger of empiric antimicrobial therapy for nosocomial SBP. Hepatology. 2016;64:2267–8.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
National Cancer Institute. Common terminology criteria for adverse events. Bethesda: National Institutes of Health; 2009.
Fukuhara S, Ware JE Jr, Kosinski M, et al. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol. 1998;51:1045–53.
Dimenäs E, Glise H, Hallerbäck B, et al. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681–7.
Morita T, Fujimoto K, Namba M, et al. Palliative care needs of cancer outpatients receiving chemotherapy: an audit of a clinical screening project. Support Care Cancer. 2008;16:101–7.
Fukazawa R, Koyama S, Kanetaka H, et al. Leg edema detected on comprehensive geriatric assessment for elderly outpatients and its associated risk factors. Nihon Ronen Igakkai Zasshi. 2013;50:384–91.
National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59:1–232.
Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228:303–8.
Hesterberg TC. What teachers should know about the bootstrap: resampling in the undergraduate statistics curriculum. Am Stat. 2015;69:371–86.
Sakaida I, Terai S, Kurosaki M, et al. Effectiveness and safety of tolvaptan in liver cirrhosis patients with edema: interim results of post-marketing surveillance of tolvaptan in liver cirrhosis (START study). Hepatol Res. 2016;. doi:10.1111/hepr.12852 (Epub ahead of print).
Sakaida I, Okita K. Correlation between changes in bodyweight and changes in ascites volume in liver cirrhosis patients with hepatic edema in short-term diuretic therapy. Hepatol Res. 2014;44:735–9.
Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–76.
Higginson IJ, McCarthy M. Validity of the support team assessment schedule: do staffs’ ratings reflect those made by patients or their families? Palliat Med. 1993;7:219–28.
Onishi Y, Wakita T, Fukuhara S, et al. Development and validation of a symptom scale specific for ascites accompanied with cirrhosis: the ASI-7. Clin Transl Gastroenterol. 2014;5:e48.
Younossi ZM, Guyatt G, Kiwi M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300.
Nakamura T, Sata M, Hiroishi K, et al. Contribution of diuretic therapy with human serum albumin to the management of ascites in patients with advanced liver cirrhosis: a prospective cohort study. Mol Clin Oncol. 2014;2:349–55.
Felker GM, Mentz RJ, Cole R, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2016;. doi:10.1016/j.jacc.2016.09.004 (Epub ahead of print).
Author information
Authors and Affiliations
Contributions
YH, HU, HN, AH, TI, MK, ST, HY, KO, and KK conceived of the study and participated in its design and coordination. YH, HU, HN, AH, MK, HK, and TI conducted the data analysis. YH interpreted the findings and drafted the original manuscript. HU, HY, ST, KO, and KK edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
HN, ST, IS, NI, KO and KK have received honoraria from Otsuka Pharmaceutical Co., Ltd. The other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hiramine, Y., Uojima, H., Nakanishi, H. et al. Response criteria of tolvaptan for the treatment of hepatic edema. J Gastroenterol 53, 258–268 (2018). https://doi.org/10.1007/s00535-017-1366-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1366-6