Abstract
Background
Hepatitis C virus (HCV) infection is common in hemodialysis patients and worsens their prognosis, while antiviral therapy options are limited. Recently, clinical trial and real-world, small-scale studies have reported excellent responses to direct-acting antivirals in patients with advanced chronic kidney diseases. However, real-world, large-scale data were lacking. This large multicenter analysis included HCV-infected hemodialysis patients receiving combination therapy with a nonstructural protein 5A (NS5A) inhibitor, daclatasvir (DCV), and a protease inhibitor, asunaprevir (ASV).
Methods
Twenty-three centers in Japan participated in this study of 123 hemodialysis patients with genotype 1 HCV infection, who received DCV/ASV combination therapy between November 2014 and March 2016. We collected and analyzed data relating to treatment outcome, baseline clinical information, laboratory measurements (during and after the treatment), and adverse events.
Results
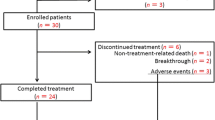
Thirty-nine patients (31.7%) had advanced liver fibrosis, 12 (9.8%) had histories of hepatocellular carcinoma (HCC), and 18 (14.6%) had baseline resistance-associated variants (RAVs) of NS5A. The overall sustained virological response (SVR)12 rate was 95.9% (118/123). Notably, all patients with HCC and 94.4% (17/18) of those with NS5A RAVs achieved SVR12. Significant factors associated with non-SVR were advanced fibrosis and the interleukin-28B non-TT genotype at rs8099917. Four patients (3.3%) discontinued therapy because of adverse events including elevated serum alanine transaminase levels (n = 2), rash (n = 1), and HCC (n = 1); all of these achieved SVR12.
Conclusions
This real-world, nationwide study revealed that DCV/ASV combination therapy was safe and highly effective for hemodialysis patients with genotype 1 HCV infections. This study was registered at the UMIN Clinical Trials Registry (UMIN000024227).


Similar content being viewed by others
Abbreviations
- ASV:
-
Asunaprevir
- CHC:
-
Chronic hepatitis C
- DAAs:
-
Direct-acting antivirals
- DCV:
-
Daclatasvir
- DM:
-
Diabetes mellitus
- ESRD:
-
End-stage renal disease
- HCV:
-
Hepatitis C virus
- HD:
-
Hemodialysis
- RAVs:
-
Resistance-associated variants
- SVR:
-
Sustained virological response
References
Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42.
Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46.
Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57.
Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15(6):645–53.
Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21(11):762–8.
Kumada H, Hayashi N, Izumi N, et al. Simeprevir (TMC435) once daily with peginterferon-alpha-2b and ribavirin in patients with genotype 1 hepatitis C virus infection: the CONCERTO-4 study. Hepatol Res. 2015;45(5):501–13.
Sciences G. SOVALDI® (sofosbuvir) tablets, for oral use: PRESCRIBING INFORMATION. 2015.
Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–45.
Pockros PJ, Reddy KR, Mantry PS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150(7):1590–8.
Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59(6):2083–91.
Suda G, Kudo M, Nagasaka A, et al. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol. 2016;51:733–40.
Toyoda H, Kumada T, Tada T, et al. Safety and efficacy of dual direct-acting antiviral therapy (daclatasvir and asunaprevir) for chronic hepatitis C virus genotype 1 infection in patients on hemodialysis. J Gastroenterol. 2016;51(7):741–7.
Kawakami Y, Imamura M, Ikeda H, et al. Pharmacokinetics, efficacy and safety of daclatasvir plus asunaprevir in dialysis patients with chronic hepatitis C: pilot study. J Viral Hepat. 2016;23:850–6.
Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6.
Ohnishi Y, Tanaka T, Ozaki K, et al. A high-throughput SNP typing system for genome-wide association studies. J Hum Genet. 2001;46(8):471–7.
Suzuki A, Yamada R, Chang X, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34(4):395–402.
Uchida Y, Kouyama J, Naiki K, et al. A novel simple assay system to quantify the percent HCV-RNA levels of NS5A Y93H mutant strains and Y93 wild-type strains relative to the total HCV-RNA levels to determine the indication for antiviral therapy with NS5A inhibitors. PLoS One. 2014;9(11):e112647.
Ito J, Suda G, Yamamoto Y, Nagasaka A, et al. Prevalence and characteristics of naturally occurring sofosbuvir resistance-associated variants in patients with hepatitis C virus genotype 1b infection. Hepatol Res. 2016;46:1294–1303.
Ogawa E, Furusyo N, Yamashita N, et al. Effectiveness and safety of daclatasvir plus asunaprevir for HCV genotype 1b patients aged 75 and over with or without cirrhosis. Hepatol Res. 2016;47:E120–E131.
Fabrizi F, Plaisier E, Saadoun D, et al. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61(4):623–37.
Lee JJ, Lin MY, Chang JS, et al. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9(6):e100790.
Iwasa Y, Otsubo S, Sugi O, et al. Patterns in the prevalence of hepatitis C virus infection at the start of hemodialysis in Japan. Clin Exp Nephrol. 2008;12(1):53–7.
Bergman S, Accortt N, Turner A, et al. Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis. 2005;45(4):684–9.
Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18(5):1584–93.
Nakayama E, Akiba T, Marumo F, et al. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11(10):1896–902.
Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol. 2003;14(12):3270–7.
Fabrizi F, Takkouche B, Lunghi G, et al. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14(10):697–703.
Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29(1):257–63.
Akiba T, Hora K, Imawari M, et al. 2011 Japanese Society for Dialysis Therapy guidelines for the treatment of hepatitis C virus infection in dialysis patients. Ther Apher Dial. 2012;16(4):289–310.
Kidney Disease: Improving Global Outcomes KDIGO. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;109:S1–99.
Gordon CE, Uhlig K, Lau J, et al. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51(2):263–77.
Fabrizi F, Dixit V, Messa P, et al. Pegylated interferon mono-therapy of chronic hepatitis C in the dialysis population: systematic review and meta-analysis. Ther Apher Dial. 2015;19(6):611–21.
Liu CH, Liang CC, Huang KW, et al. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol. 2011;6(5):1057–65.
Fridell RA, Wang C, Sun JH, et al. Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations. Hepatology. 2011;54(6):1924–35.
Zeuzem S, Soriano V, Asselah T, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369(7):630–9.
Akuta N, Sezaki H, Suzuki F, et al. Retreatment efficacy and predictors of ledipasvir plus sofosbuvir to HCV genotype 1 in Japan. J Med Virol. 2017;89(2):284–90.
Meissner EG, Bon D, Prokunina-Olsson L, et al. IFNL4-DeltaG genotype is associated with slower viral clearance in hepatitis C, genotype-1 patients treated with sofosbuvir and ribavirin. J Infect Dis. 2014;209(11):1700–4.
Ono K, Ono T, Matsumata T. The pathogenesis of decreased aspartate aminotransferase and alanine aminotransferase activity in the plasma of hemodialysis patients: the role of vitamin B6 deficiency. Clin Nephrol. 1995;43(6):405–8.
Matsumoto N, Ikeda H, Shigefuku R, et al. Hemoglobin decrease with iron deficiency induced by daclatasvir plus asunaprevir combination therapy for chronic hepatitis C virus genotype 1b. PLoS One. 2016;11(3):e0151238.
Asahina Y, Tsuchiya K, Nishimura T, et al. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58(4):1253–62.
Foley RN, Parfrey PS, Harnett JD, et al. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7(5):728–36.
Lok AS, Gardiner DF, Hezode C, et al. Randomized trial of daclatasvir and asunaprevir with or without PegIFN/RBV for hepatitis C virus genotype 1 null responders. J Hepatol. 2014;60(3):490–9.
Acknowledgements
The authors would like to thank all of the patients and their families, as well as the investigators and staff of the 23 participating institutions.
Author information
Authors and Affiliations
Contributions
GS, NF, HT, YK designed the study, and wrote the initial draft of the manuscript. NS contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. The final version of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
N. Sakamoto received lecture fees from Bristol Myers Squibb and Pharmaceutical K.K., grants and endowments from MSD K.K. and Chugai Pharmaceutical Co., Ltd., and a research grant from Gilead Sciences, Inc. G. Suda received research grants from Bristol Myers Squibb. K. Chayama has received research grants and consulting fees from Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Mitsubishi Tanabe Pharma, Daiichi Sankyo, Toray Industries, Otsuka Pharmaceutical Company, and GlaxoSmithKline K.K. Y. Ueno has received research grants from Bristol-Myers Squibb, MSD K.K., Gilead Sciences, Inc., and AbbVie G.K. K. Furuya has received lecture fees from Bristol-Myers Squibb and MSD K.K. H. Toyoda and T. Kumada received research grants from Bristol Myers Squibb. K. Takaguchi has received lecture fees from Bristol-Myers Squibb. Y. Tanaka have received lecture fees from Bristol-Myers Squibb, MSD K.K., Chugai Pharmaceutical Co., Ltd., AbbVie Inc., Janssen Pharmaceutical K.K., Gilead Sciences, and a research grant from Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd,, and AbbVie Inc. The other authors have nothing to disclose.
Funding
This study was supported in part by grants from the Japan Agency for Medical Research and Development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2017_1353_MOESM1_ESM.tif
Correlation between the FIB4 index and FibroScan value. The correlation between the baseline FIB4 index and FibroScan value was analyzed by Pearson’s rank test (n = 68). (TIFF 5199 kb)
Rights and permissions
About this article
Cite this article
Suda, G., Furusyo, N., Toyoda, H. et al. Daclatasvir and asunaprevir in hemodialysis patients with hepatitis C virus infection: a nationwide retrospective study in Japan. J Gastroenterol 53, 119–128 (2018). https://doi.org/10.1007/s00535-017-1353-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1353-y