Abstract
Background
Superficial non-ampullary duodenal epithelial tumors (SNADETs) are relatively rare, but they are now being detected more frequently due to advances in endoscopic technology. Nevertheless, the pathological nature of SNADETs remains unclear and a management strategy for these tumors has not been established.
Methods
To elucidate the clinicopathological features, we conducted a retrospective analysis of 138 endoscopically resected SNADETs. Lesions were classified into two groups by histological grade according to the Vienna classification: category 3 (71 lesions, 51.4%) and category 4/5 (67 lesions, 48.6%).
Results
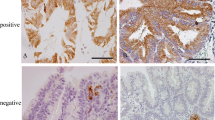
Compared with category 3 lesions, category 4/5 lesions were significantly more common in elderly patients (p < 0.001) and had a significantly larger tumor diameter (p = 0.001). Immunohistochemical analysis showed that category 4/5 lesions expressed MUC5AC (p = 0.002), MUC6 (p < 0.001), and p53 (p = 0.003) significantly more frequently and expressed CD10 (p = 0.002) and CDX2 (p = 0.029) significantly less frequently. Multivariate regression analysis showed that advanced age (p < 0.001), MUC6 expression (p = 0.001), and p53 expression (p = 0.004) were independent risk factors for a classification of category 4/5. In addition, advanced age (p = 0.010) and MUC5AC expression (p = 0.011) were identified as risk factors for lesions classified as category 4.2 (noninvasive carcinoma) or higher. All category 5 lesions expressed MUC5AC.
Conclusions
The gastric phenotype of MUC5AC and MUC6 may be linked to the malignant potential of SNADETs.

Similar content being viewed by others
References
Endo M, Abiko Y, Oana S, et al. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010;22:360–5.
Alwmark A, Andersson A, Lasson A. Primary carcinoma of the duodenum. Ann Surg. 1980;191:13–8.
Jepsen JM, Persson M, Jakobsen NO, et al. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483–7.
Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19:58–69.
Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc. 2014;26(Suppl 2):23–9.
Howe JR, Karnell LH, Menck HR, et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985–1995. Cancer. 1999;86:2693–706.
Kikuchi D, Hoteya S, Iizuka T, et al. Diagnostic algorithm of magnifying endoscopy with narrow band imaging for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26(Suppl 2):16–22.
Hoteya S, Yahagi N, Iizuka T, et al. Endoscopic submucosal dissection for nonampullary large superficial adenocarcinoma/adenoma of the duodenum: feasibility and long-term outcomes. Endosc Int Open. 2013;1:2–7.
Tsuji S, Doyama H, Tsuji K, et al. Preoperative endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors, including magnifying endoscopy. World J Gastroenterol. 2015;21:11832–41.
Hoteya S, Kaise M, Iizuka T, et al. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: analysis of risk factors. Dig Endosc. 2015;27:323–30.
Hoteya S, Furuhata T, Takahito T, et al. Endoscopic submucosal dissection and endoscopic mucosal resection for non-ampullary superficial duodenal tumor. Digestion. 2017;95:36–42.
Inoue T, Uedo N, Yamashina T, et al. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220–7.
Kakushima N, Kanemoto H, Tanaka M, et al. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501–8.
Oka S, Tanaka S, Nagata S, et al. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381–6.
Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–1.
Liu XP, Kawauchi S, Oga A, et al. Combined examination of p27(Kip1), p21(Waf1/Cip1) and p53 expression allows precise estimation of prognosis in patients with gastric carcinoma. Histopathology. 2001;39:603–10.
Boonla C, Wongkham S, Sheehan JK, et al. Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer. 2003;98:1438–43.
Kocer B, Soran A, Erdogan S, et al. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470–7.
Shibahara H, Higashi M, Koriyama C, et al. Pathobiological implications of mucin (MUC) expression in the outcome of small bowel cancer. PLoS One. 2014;9:e86111.
Imai Y, Yamagishi H, Fukuda K, et al. Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J Gastroenterol. 2013;19:3957–68.
Kolodziejczyk P, Yao T, Oya M, et al. Long-term follow-up study of patients with gastric adenomas with malignant transformation. An immunohistochemical and histochemical analysis. Cancer. 1994;74:2896–907.
Hida R, Yamamoto H, Hirahashi M, et al. Duodenal neoplasms of gastric phenotype: an immunohistochemical and genetic study with a practical approach to the classification. Am J Surg Pathol. 2017;41:343–53.
Amaya S, Sasaki M, Watanabe Y, et al. Expression of MUC1 and MUC2 and carbohydrate antigen Tn change during malignant transformation of biliary papillomatosis. Histopathology. 2001;38:550–60.
Baker SJ, Fearon ER, Nigro JM, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244:217–21.
Rodrigues NR, Rowan A, Smith ME, et al. p53 mutations in colorectal cancer. Proc Natl Acad Sci. 1990;87:7555–9.
Acknowledgments
The authors would like to thank the medical technologists of the Department of Pathology for technical assistance in this study. This study was supported by funding from Toranomon Hospital Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Toba, T., Inoshita, N., Kaise, M. et al. Clinicopathological features of superficial non-ampurally duodenal epithelial tumor; gastric phenotype of histology correlates to higher malignant potency. J Gastroenterol 53, 64–70 (2018). https://doi.org/10.1007/s00535-017-1327-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1327-0