Abstract
Background
This exploratory trial was conducted to investigate whether daikenchuto accelerates the recovery of gastrointestinal function in patients undergoing open surgery for sigmoid or rectosigmoid cancer.
Methods
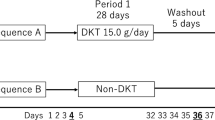
Eighty-eight patients who underwent colectomy at one of the 11 clinical trial sites in Japan from January 2009 to June 2011 were registered in the study. Patients received either placebo or daikenchuto (15.0 g/day, 5 g three times a day) from postoperative day 2 to postoperative day 8. The study end points included the gastrointestinal tract transit time evaluated with radiopaque markers and the time to first flatus. The safety profile of daikenchuto was also evaluated until postoperative day 8.
Results
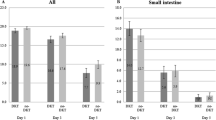
Seventy-one patients (daikenchuto, n = 38; placebo, n = 33) were statistically analyzed. Although the number of radiopaque markers in the anal side of the small intestine at 6 h was significantly greater in the daikenchuto group than in the placebo group (15.19 vs 10.06, p = 0.008), the total transit analysis results and the mean time to first flatus did not differ significantly between the two groups.
Conclusions
Daikenchuto has a positive effect on the resolution of delayed gastric emptying, but has a limited effect on the resolution of postoperative paralytic ileus after open surgery in patients with sigmoid or rectosigmoid cancer. Daikenchuto may contribute to early oral intake in the postoperative course.



Similar content being viewed by others
References
Center for Cancer Control and Information Services, National Cancer Center, Japan, 2012.
Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg. 2011;254:868–75.
van Bree SH, Vlug MS, Bemelman WA, et al. Faster recovery of gastrointestinal transit after laparoscopy and fast-track care in patients undergoing colonic surgery. Gastroenterology. 2011;141(3):872–80.e1–4.
Livingston EH, Passaro EP Jr. Postoperative ileus. Dig Dis Sci. 1990;35:121–32.
Kraft M, Maclaren R, Du W, et al. Alvimopan (Entereg) for the management of postoperative ileus in patients undergoing bowel resection. P T. 2010;35:44–9.
Brown TA, McDonald J, Williard W. A prospective, randomized, double-blinded, placebo-controlled trial of cisapride after colorectal surgery. Am J Surg. 1999;177:399–401.
Longo WE, Vernava AM 3rd. Prokinetic agents for lower gastrointestinal motility disorders. Dis Colon Rectum. 1993;36:696–708.
Yoshikawa K, Shimada M, Nishioka M, et al. The effects of the Kampo medicine (Japanese herbal medicine) “Daikenchuto” on the surgical inflammatory response following laparoscopic colorectal resection. Surg Today. 2012;42:646–51.
Furukawa Y, Shiga Y, Hanyu N. Effect of Chinese herbal medicine on gastrointestinal motility and bowel obstruction. Jpn J Gastroenterol Surg. 1995;28:956–60.
Itoh T, Yamakawa J, Mai M, et al. The effect of the herbal medicine dai-kenchu-to on post-operative ileus. J Int Med Res. 2002;30:428–32.
Takeda T, Kamiura S, Kimura T. Effectiveness of the herbal medicine daikenchuto for radiation-induced enteritis. J Altern Complement Med. 2008;14:753–5.
Jin XL, Shibata C, Naito H, et al. Intraduodenal and intrajejunal administration of the herbal medicine, dai-kenchu-tou, stimulates small intestinal motility via cholinergic receptors in conscious dogs. Dig Dis Sci. 2001;46:1171–6.
Nakamura T, Ohya M, Ishikawa H. Total and segmental colonic transit times in patients after anterior resection of sigmoid colon and rectal cancer—their relationship with postoperative abdominal symptoms and anal function. J Jpn Soc Coloproctol. 1997;50:17–32.
Satoh K, Kase Y, Yuzurihara M, et al. Effect of Dai-kenchu-to (Da-Jian-Zhong-Tang) on the delayed intestinal propulsion induced by chlorpromazine in mice. J Ethnopharmacol. 2003;86:37–44.
Tokita Y, Yuzurihara M, Sakaguchi M, et al. The pharmacological effects of Daikenchuto, a traditional herbal medicine, on delayed gastrointestinal transit in rat postoperative ileus. J Pharmacol Sci. 2007;104:303–10.
Satoh K, Hayakawa T, Kase Y, et al. Mechanisms for contractile effect of Dai-kenchu-to in isolated guinea pig ileum. Dig Dis Sci. 2001;46:250–6.
Endo M, Hori M, Ozaki H, et al. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. J Gastroenterol. 2014;49:1026–39.
Manabe N, Camilleri M, Rao A, et al. Effect of daikenchuto (TU-100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298:G970–5.
Shimada M, Morine Y, Nagano H, et al. Effect of TU-100, a traditional Japanese medicine, administered after hepatic resection in patients with liver cancer: a multi-center, phase III trial (JFMC40-1001). Int J Clin Oncol. 2015;20:95–104.
Arhan P, Devroede G, Jehannin B, et al. Segmental colonic transit time. Dis Colon Rectum. 1981;24:625–9.
Kono T, Kanematsu T, Kitajima M. Exodus of Kampo, traditional Japanese medicine, from the complementary and alternative medicines: is it time yet? Surgery. 2009;146:837–40.
Matsuoka H, Maeda K, Katsuno H, et al. Recovery of upper gastrointestinal bowel movement after rectosigmoid cancer surgery: a pilot transit analysis. Int Surg. 2011;96:281–5.
Hinton JM, Lennard-Jones JE, Young AC. A new method for studying gut transit times using radioopaque markers. Gut. 1969;10:842–7.
Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–7.
Krevsky B, Malmud LS, D’Ercole F, et al. Colonic transit scintigraphy. A physiologic approach to the quantitative measurement of colonic transit in humans. Gastroenterology. 1986;91:1102–12.
van der Sijp JR, Kamm MA, Nightingale JM, et al. Radioisotope determination of regional colonic transit in severe constipation: comparison with radio opaque markers. Gut. 1993;34:402–8.
Iida M, Ikeda M, Kishimoto M, et al. Evaluation of gut motility in type II diabetes by the radiopaque marker method. J Gastroenterol Hepatol. 2000;15:381–5.
Chan YK, Kwan AC, Yuen H, et al. Normal colon transit time in healthy Chinese adults in Hong Kong. J Gastroenterol Hepatol. 2004;19:1270–5.
Chaussade S, Roche H, Khyari A, et al. Measurement of colonic transit time: description and validation of a new method. Gastroenterol Clin Biol. 1986;10:385–9.
Iizuka I, Koda K, Seike K, et al. Defecatory malfunction caused by motility disorder of the neorectum after anterior resection for rectal cancer. Am J Surg. 2004;188:176–80.
Sato Y, Katagiri F, Inoue S, et al. Dai-kenchu-to raises levels of calcitonin gene-related peptide and substance P in human plasma. Biol Pharm Bull. 2004;27:1875–7.
Kono T, Kaneko A, Omiya Y, et al. Epithelial transient receptor potential ankyrin 1 (TRPA1)-dependent adrenomedullin upregulates blood flow in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304:G428–36.
Kono T, Koseki T, Chiba S, et al. Colonic vascular conductance increased by Daikenchuto via calcitonin gene-related peptide and receptor-activity modifying protein 1. J Surg Res. 2008;150:78–84.
Mochiki E, Yanai M, Ohno T, et al. The effect of traditional Japanese medicine (Kampo) on gastrointestinal function. Surg Today. 2010;40:1105–11.
Endo S, Nishida T, Nishikawa K, et al. Dai-kenchu-to, a Chinese herbal medicine, improves stasis of patients with total gastrectomy and jejunal pouch interposition. Am J Surg. 2006;192:9–13.
Yasunaga H, Miyata H, Horiguchi H, et al. Effect of the Japanese herbal kampo medicine dai-kenchu-to on postoperative adhesive small bowel obstruction requiring long-tube decompression: a propensity score analysis. Evid Based Complement Altern Med. 2011;2011:264289.
Tsunoda A, Shibusawa M, Takata M, et al. Early oral feeding should be resumed following the resolution of gastric ileus. Hepatogastroenterology. 2005;63:775–9.
Vather R, Josephson R, Jaung R, et al. Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery. 2015;157:764–73.
Chapuis PH, Bokey L, Keshava A, et al. Risk factors for prolonged ileus after resection of colorectal cancer. Ann Surg. 2013;257:909–15.
Acknowledgment
This study was supported by the Japanese Foundation for Multidisciplinary Treatment of Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Katsuno, H., Maeda, K., Ohya, M. et al. Clinical pharmacology of daikenchuto assessed by transit analysis using radiopaque markers in patients with colon cancer undergoing open surgery: a multicenter double-blind randomized placebo-controlled study (JFMC39-0902 additional study). J Gastroenterol 51, 222–229 (2016). https://doi.org/10.1007/s00535-015-1100-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1100-1