Abstract
Background
Patients with ulcerative colitis (UC) exhibit overproduction of reactive oxygen species (ROS) and imbalance of colonic microflora. We previously developed a novel redox nanoparticle (RNPO), which effectively scavenged ROS in the inflamed mucosa of mice with dextran sodium sulfate (DSS)-induced colitis after oral administration. The objective of this study was to examine whether the orally administered RNPO changed the colonic microflora in healthy mice and those with colitis.
Methods
RNPO was synthesized by self-assembly of an amphiphilic block copolymer that contains stable nitroxide radicals in hydrophobic side chain via ether linkage. Colitis was induced in mice by supplementing DSS in drinking water for 7 days, and RNPO was orally administered daily during DSS treatment. The alterations of fecal microflora during treatment of DSS and RNPO were investigated using microbiological assays.
Results
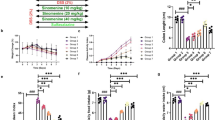
We investigated that RNPO did not result in significant changes to the fecal microflora in healthy mice. Although total aerobic and anaerobic bacteria were not significantly different between experimental groups, a remarkable increase in commensal bacteria (Escherichia coli and Staphylococcus sp.) was observed in mice with DSS-induced colitis. Interestingly, orally administered RNPO remarkably reduced the rate of increase of these commensal bacteria in mice with colitis.
Conclusions
On the basis of the obtained results, it was confirmed that the oral administration of RNPO did not change any composition of bacteria in feces, which strongly suggests a protective effect of RNPO on healthy environments in intestinal microflora. RNPO may become an effective and safe medication for treatment of UC.






Similar content being viewed by others
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29.
Edward VL. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17.
Simmonds NJ, Rampton DS. Inflammatory bowel disease: a radical view. Gut. 1993;34:865–8.
Babbs CF. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992;13:169–81.
Gionchetti P, Rizzello F, Lammers KM, Morselli C, Sollazzi L, Davies S, et al. Antibiotics and probiotics in treatment of inflammatory bowel disease. World J Gastroenterol. 2006;12:3306–13.
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nat Rev. 2011;44:307–17.
Sartor RB. The influence of normal microbial flora on the development of chronic inflammation. Res Immunol. 1997;148:567–76.
Strauch UG, Obermeier F, Grunwald N, Gürster S, Dunger N, Schultz M, et al. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54:1546–52.
Gibson GR, Roberfroid M. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12.
Sasaki M, Klapproth J. The role of bacteria in the pathogenesis of ulcerative colitis. J Signal Transduct. 2012; doi: 10.1155/2012/704953.
Araki Y, Andoh A, Tsujikawa T, Fujiyama Y, Bamba T. Alterations in intestinal microflora, faecal bile acids and short chain fatty acids in dextran sulphate sodium-induced experimental acute colitis in rats. Eur J Gastroenterol Hepatol. 2011;13:107–12.
Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143:1027–36.e3.
Yoshitomi T, Miyamoto D, Nagasaki Y. Design of core-shell-type nanoparticles carrying stable radicals in the core. Biomacromolecules. 2009;10:596–601.
Yoshitomi T, Nagasaki Y. Nitroxyl radical-containing nanoparticles for novel nanomedicine against oxidative stress injury. Nanomedicine. 2011;6:509–18.
Solomon L, Mansor S, Mallon P, Donnelly E, Hoper M, Loughrey M, et al. The dextran sulphate sodium (DSS) model of colitis: an overview. Comp Clin Pathol. 2010;19:235–9.
Van der Waaij LA, Harmsen HJ, Madjipour M, Kroese FG, Zwiers M, van Dullemen HM, et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm Bowel Dis. 2005;10:865–71.
Xia Y, Chen HQ, Zhang M, Jiang YQ, Hang XM, Qin HL. Effect of Lactobacillus plantarum LP-Only on gut flora and colitis in interleukin-10 knockout mice. J Gastroenterol Hepatol. 2011;26:405–11.
Vereecke L, Sze M, Mc Guire C, Rogiers B, Chu Y, Schmidt-Supprian M, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–23.
Friend DR, Sellin J. Drug delivery in advancing the treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57:215–6.
Hanauer SB. Medical therapy for ulcerative colitis 2004. Gastroenterology. 2004;126:1582–92.
Swidsinski A, Loening-Baucke V, Bengmark S, Lochs H, Dörffel Y. Azathioprine and mesalazine-induced effects on the mucosal flora in patients with IBD colitis. Inflamm Bowel Dis. 2007;1:51–6.
West B, Lendrum R, Hill MJ, Walker G. Effects of sulphasalazine (Salazopyrin) on faecal flora in patients with inflammatory bowel disease. Gut. 1974;15:960–5.
Singh K, Chaturvedi R, Barry DP, Coburn LA, Asim M, Lewis ND, et al. The apolipoprotein E-mimetic peptide COG112 inhibits NF-kappaB signaling, proinflammatory cytokine expression, and disease activity in murine models of colitis. J Biol Chem. 2011;286:3839–50.
Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–19.
Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut. 2003;52:988–97.
Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2 + D phylogenetic group in inflammatory bowel disease. Gut. 2007;56:669–75.
Lu J, Wang A, Ansari S, Hershberg RM, McKay DM. Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology. 2003;125:1785–95.
Vesterlund S, Karp M, Salminen S, Ouwehand AC. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology. 2006;152:1819–26.
Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–72.
Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–37.
Hans W, Schölmerich J, Gross V, Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur J Gastroenterol Hepatol. 2000;12:267–73.
Johansson ME, Gustafsson JK, Sjöberg KE, Petersson J, Holm L, Sjövall H, et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE. 2010;5:e12238.
Zhou FX, Chen L, Liu XW, Ouyang CH, Wu XP, Wang XH, et al. Lactobacillus crispatus M206119 exacerbates murine DSS-colitis by interfering with inflammatory responses. World J Gastroenterol. 2012;18:2344–56.
Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–54.
Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–22.
Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–24.
Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, MacDermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94:04–12.
Brownlee IA, Knight J, Dettmar PW, Pearson JP. Action of reactive oxygen species on colonic mucus secretions. Free Radic Biol Med. 2007;43:800–8.
Acknowledgments
A portion of this work was supported by a Grant-in-Aid for Scientific Research A (No. 21240050) and the World Premier International Research Center Initiative (WPI Initiative) on Materials Nanoarchitronics of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Conflict of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity having a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed. The authors declare that they have no conflicts of interest with this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vong, L.B., Yoshitomi, T., Morikawa, K. et al. Oral nanotherapeutics: effect of redox nanoparticle on microflora in mice with dextran sodium sulfate-induced colitis. J Gastroenterol 49, 806–813 (2014). https://doi.org/10.1007/s00535-013-0836-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0836-8