Abstract
Background
ATP-sensitive potassium (K-ATP) channels couple cellular metabolism to electric activity. Although Kir6.2-composed K-ATP channel (Kir6.2/K-ATP channel) has been demonstrated to regulate inflammation, a common cause of most liver diseases, its role in liver injury remains elusive.
Methods
Kir6.2 knockout mice were used to prepared LPS-induced liver injury model so as to investigate the role of Kir6.2/K-ATP channels in the injury. Histochemistry was applied to evaluate the extent of liver injury. Proinflammatory cytokines were analyzed by ELISA. Endoplasmic reticulum (ER) stress and autophagy were assessed by western blotting.
Results
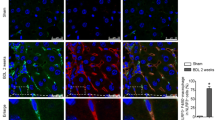
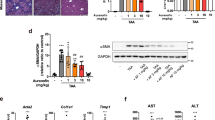
We showed that Kir6.2 knockout markedly promoted the infiltration of lymphocytes and neutrophils in liver and significantly elevated serum levels of alanine transaminase (ALT) in respond to LPS treatment. We further found that Kir6.2 deficiency enhanced the activation of NF-κB and NLRP3 inflammasome following LPS challenge, and thereby increased the levels of pro-inflammatory cytokines IL-1β, IL-18 and TNF-α. Treatment of wild-type mice with the K-ATP channel opener iptakalim (IPT) could protect against LPS-induced liver injury through attenuating NLRP3 inflammasome-mediated inflammatory responses. Furthermore, Kir6.2 knockout-induced activation of NLRP3 inflammasome aggravated endoplasmic reticulum (ER) stress, autophagy and subsequent hepatocyte death.
Conclusion
Kir6.2 deficiency exacerbated LPS-induced liver injury by enhancing NLRP3 inflammasome-mediated inflammatory response. Thus, Kir6.2/K-ATP channel may be a potential candidate target for the treatment and prevention of liver injury.







Similar content being viewed by others
References
Morrell MR, Micek ST, Kollef MH. The management of severe sepsis and septic shock. Infect Dis Clin North Am. 2009;23:485–501.
Nath B, Szabo G. Alcohol-induced modulation of signaling pathways in liver parenchymal and nonparenchymal cells: implications for immunity. Semin Liver Dis. 2009;29:166–77.
Michel O. Role of lipopolysaccharide (LPS) in asthma and other pulmonary conditions. J Endotoxin Res. 2003;9:293–300.
Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology. 2009;136:1476–83.
Dare AJ, Phillips AR, Hickey AJ, Mittal A, Loveday B, Thompson N, et al. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radical Biol Med. 2009;47:1517–25.
Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–63.
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85.
Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220–34.
Watanabe A, Sohail MA, Gomes DA, Hashmi A, Nagata J, Sutterwala FS, et al. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol. 2009;296:G1248–57.
Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642–54.
Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7.
Imamura M, Tsutsui H, Yasuda K, Uchiyama R, Yumikura-Futatsugi S, Mitani K, et al. Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J Hepatol. 2009;51:333–41.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32.
Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Investig. 2012;122:3476–89.
Hoque R, Vodovotz Y, Mehal W. Therapeutic strategies in inflammasome mediated diseases of the liver. J Hepatol. 2013;58:1047–52.
Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K + channels. Prog Biophys Mol Biol. 2003;81:133–76.
Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K + channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol. 1998;274:C25–37.
Sikka P, Kapoor S, Bindra VK, Saini M, Saxena KK. Iptakalim: a novel multi-utility potassium channel opener. J Pharmacol Pharmacother. 2012;3:12–4.
Buckley JF, Singer M, Clapp LH. Role of KATP channels in sepsis. Cardiovasc Res. 2006;72:220–30.
Hai S, Takemura S, Minamiyama Y, Yamasaki K, Yamamoto S, Kodai S, et al. Mitochondrial K(ATP) channel opener prevents ischemia–reperfusion injury in rat liver. Transplant Proc. 2005;37:428–31.
Zhou F, Yao HH, Wu JY, Ding JH, Sun T, Hu G. Opening of microglial K(ATP) channels inhibits rotenone-induced neuroinflammation. J Cell Mol Med. 2008;12:1559–70.
Plachinta RV, de Klaver MJ, Hayes JK, Rich GF. The protective effect of protein kinase C and adenosine triphosphate-sensitive potassium channel agonists against inflammation in rat endothelium and vascular smooth muscle in vitro and in vivo. Anesth Analg. 2004;99:556–61.
Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA. 1998;95:10402–6.
Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(323–34):e7.
Eijo G, Zarate S, Jaita G, Ferraris J, Magri ML, Zaldivar V, et al. Inhibition of nuclear factor-kappa B sensitises anterior pituitary cells to tumour necrosis factor-alpha- and lipopolysaccharide-induced apoptosis. J Neuroendocrinol. 2011;23:651–9.
Ganz M, Csak T, Nath B, Szabo G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol. 2011;17:4772–8.
Tsutsui H, Imamura M, Fujimoto J, Nakanishi K. The TLR4/TRIF-mediated activation of nlrp3 inflammasome underlies endotoxin-induced liver injury in mice. Gastroenterol Res Pract. 2010;2010:641865.
Rautou PE, Cazals-Hatem D, Moreau R, Francoz C, Feldmann G, Lebrec D, et al. Acute liver cell damage in patients with anorexia nervosa: a possible role of starvation-induced hepatocyte autophagy. Gastroenterology. 2008;135:840–8, 8 e1–3.
Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–44.
Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–74.
Yamamoto M, Yaginuma K, Tsutsui H, Sagara J, Guan X, Seki E, et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells. 2004;9:1055–67.
Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–32.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91.
Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74.
Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809.
Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–77.
Ben Mosbah I, Alfany-Fernandez I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, et al. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia–reperfusion. Cell Death Dis. 2010;1:e52.
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77.
Sanges D, Marigo V. Cross-talk between two apoptotic pathways activated by endoplasmic reticulum stress: differential contribution of caspase-12 and AIF. Apoptosis. 2006;11:1629–41.
Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012;110:174–89.
Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8.
Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32:431–6.
Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–96.
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30.
Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J. 2011;30:4701–11.
Vernon PJ, Tang D. Eat-me: autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid Redox Signal. 2013;18:677–91.
Acknowledgments
This work was supported by the grant from the National Key Program of Basic Research of China (No. 2009CB521906 and No. 2011CB504103), the National Natural Science Foundation of China (No. 81030060), and the National Science & Technology Major Project (No. 2012ZX09304-001). We are grateful to Drs. Susumu Seino and Takashi Miki, Kobe University, for generous donation of Kir6.2 knockout mice.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, RH., Tan, J., Yan, N. et al. Kir6.2 knockout aggravates lipopolysaccharide-induced mouse liver injury via enhancing NLRP3 inflammasome activation. J Gastroenterol 49, 727–736 (2014). https://doi.org/10.1007/s00535-013-0823-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0823-0