Abstract
Background
We investigated whether the administration of maintenance doses of interferon prevented hepatocellular carcinoma (HCC) in patients with chronic hepatitis C.
Methods
Study 1: A multicenter, retrospective, cooperative study was carried out to determine whether long-term administration of low-dose peginterferon alpha-2a (PegIFNα-2a) prevented HCC development in patients with chronic hepatitis C. In total, 594 chronic hepatitis C patients without a history of HCC were enrolled and treated with 90 μg PegIFNα-2a administered weekly or bi-weekly for at least 1 year. Study 2: HCC developed in 16 of 99 additional patients without PegIFNα-2a treatment during 3.8 years of observation. A propensity-matched control study was then carried out to compare the incidence of HCC between the 59 patients who received low-dose PegIFNα-2a (PegIFNα-2a group) and 59 patients who did not receive PegIFNα-2a treatment (control group), matched for sex, age, platelet count, and total bilirubin levels.
Results
Study 1: HCC developed in 49 patients. The risk of HCC was lower in patients with undetectable hepatitis C virus RNA, ≤40 IU/L alanine aminotransferase (ALT), or ≤10 ng/L alpha-fetoprotein (AFP) 24 weeks after the start of therapy. Study 2: The incidence of HCC was significantly lower in the PegIFNα-2a group than in the control group.
Conclusions
Low-dose and long-term maintenance administration of PegIFNα-2a decreased the incidence of HCC in patients with normalized ALT and AFP levels at 24 weeks compared with patients without normal ALT and AFP levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), the sixth most common cancer worldwide, often develops because of long-term hepatitis B or C virus infection [1, 2]. In particular, chronic hepatitis C and hepatic cirrhosis increase the risk of HCC; the annual incidence of tumor development in such patients may be as high as 2–4 % [3–5]. The incidence of HCC decreases in patients who achieve a sustained virological response (SVR) to interferon (IFN) treatment, although the incidence remains high in non-SVR patients [6–9]. A detailed analysis of HCC development revealed that chronic hepatitis C patients aged 65 years or more, especially those with advanced fibrosis of the liver, were at an increased risk of developing HCC [10]. For patients 65 years or older with advanced liver fibrosis, the dose of ribavirin is often reduced or the agent is discontinued, resulting in lower SVR rates in those with discontinuation of ribavirin. Establishing an effective treatment strategy for preventing the development of HCC is important for these high-risk patients.
Factors related to the development of HCC have been analyzed in patients who did not achieve an SVR even after IFN treatment; advanced fibrosis of the liver and high levels of serum alanine aminotransferase (ALT), and alpha-fetoprotein (AFP) are risk factors for HCC development [11, 12]. A randomized controlled trial was conducted in Western countries to determine whether combined peginterferon and ribavirin treatment with weekly administration of 90 μg peginterferon alpha-2a (PegIFNα-2a) could prevent HCC in non-responders. A 3.5-year follow up showed that administration of a maintenance dose of PegIFNα-2a did not reduce tumor incidence in these patients [13]. However, after 8.5 years of observation, the incidence of HCC was decreased among those in the PegIFNα-2a group with cirrhosis [14]. Meanwhile, Bruix et al. [15] reported that maintenance therapy with PegIFNα-2b did not prevent HCC in chronic hepatitis C patients with cirrhosis. In Japan, long-term low-dose administration of natural IFN has been reported to decrease the incidence of HCC [16]. In light of these conflicting results, investigations should be carried out in a large number of patients with chronic hepatitis C to resolve the question of whether IFN treatment prevents the development of HCC.
We carried out a multicenter retrospective cooperative study of patients with chronic hepatitis C to determine whether those treated with 90 μg PegIFNα-2a without ribavirin had a reduced incidence of HCC compared with those not treated with IFN.
Patients and methods
Study 1: analysis of risk factors for HCC in patients treated with long-term low-dose-PegIFNα-2a
In total, at 21 hepatitis centers throughout Japan, 743 patients with hepatitis C who had received 90 μg of PegIFNα-2a therapy weekly or bi-weekly for 1 year or more without having received the full dose (180 μg) since December 2003 were examined retrospectively for the development of HCC. The end of enrollment in this study was the end of December 2008 and the end of follow up was the end of December 2010. Patients with a history of HCC before the start of therapy and those with a therapy period of less than 48 weeks were excluded, leaving 594 patients who had undergone long-term administration of PegIFNα-2a for analysis. At the 21 centers involved in this study, 4,753 patients with chronic hepatitis C had been treated; Peg-IFN and ribavirin combination treatment had been administered to 3,877 patients, 743 patients had received Peg-IFN alone, and 133 patients had not agreed to receive IFN (a flow diagram of the enrollment of patients in this study is shown in Fig. 1). In the patients with Peg-IFN and ribavirin combination treatment, the SVR rate was 43.8 %; SVR was not achieved in 2,179 patients, and in 776 of these patients, the combination therapy was discontinued owing to adverse events or the patient’s choice. Patients who failed to achieve an SVR were not included in this study, because the incidence of HCC is known to be reduced even in non-responders to IFN [17].
The backgrounds of the 594 patients studied are shown in Table 1. Findings from the liver biopsies of the patients were classified according to international standards [18]. Long-term PegIFNα-2a treatment is approved by the Japanese Medical Insurance system. Written informed consent was obtained from all patients prior to participation in this study. The study design was approved by the regional ethics committees of the 21 centers involved in this study, including the Musashino Red Cross Hospital, in accordance with the Helsinki Declaration. The 743 patients treated with PegIFNα-2a alone were not indicated for Peg-IFNα and ribavirin combination therapy because of anemia or heart disease. The 133 patients who did not agree to receive IFN served as the control group (see Fig. 1). A large proportion of the 594 study patients had advanced fibrosis of the liver and active inflammation. A dose of 90 μg PegIFNα-2a was administered to 512 and 82 patients weekly and biweekly, respectively, according to the patients’ wishes. There were no significant differences between the weekly and biweekly groups in the patients’ background data (data not shown).
The median duration of follow up in the PegIFNα-2a group was 1,273 days (range 228–2,768 days) and HCC was observed in 49 of the 594 patients (Table 1). Pretreatment and on-treatment factors associated with the development of HCC were analyzed by Student’s t-test, the Mann–Whitney U-test, and the χ2 test (Table 2). Independent factors for the development of HCC were assessed by multivariate analysis using logistic regression. The incidence of HCC was analyzed according to the ALT, AFP, and hepatitis C virus (HCV) RNA levels 24 weeks after the start of PegIFNα-2a administration by using the Kaplan–Meier method. The risk of HCC was analyzed, using the Kaplan–Meier method, only in the non-responders with detectable HCV RNA during PegIFNα-2a administration by dividing them according to the ALT and AFP levels 24 weeks after the start of therapy. The incidence of HCC was compared between the patients with ALT levels of <41 IU/L and those with levels of ≥41 IU/L, and between patients with serum AFP levels of <10 ng/L and those with levels of ≥10 ng/mL at 24 weeks after starting treatment, because at most of the centers participating in the this study, the upper normal range of serum ALT is set at 40 IU/L, and the most significant difference in the incidence of HCC was observed between the PegIFNα-2a and control group with the cut-off serum ALT set at 41 IU/L and cutoff serum AFP set at 10 ng/mL, 24 weeks after starting treatment. The HCV RNA level was measured using the Amplicor Monitor method with a lower detection limit of 50 IU/L (Roche Diagnostics, Tokyo, Japan). A history of excess alcohol consumption was determined as >60 g alcohol per day in order to exclude alcoholic liver disease.
An asymptomatic carrier was defined as a patient with a serum ALT level within the normal range and minimal inflammation or fibrosis in the biopsied tissues of the liver. Chronic hepatitis was defined as mild-to-severe fibrosis of the liver according to liver biopsy [18]. The diagnosis of liver cirrhosis was based on the results of histological examination of the biopsied liver tissues.
Study 2: incidence of HCC in the PegIFNα-2a therapy and non-administration (control) groups in comparison with propensity-matched controls
Ninety-nine of the 133 chronic hepatitis C patients who had not received IFN were examined as controls; patients in this group received liver-protective agents such as glycyrrhizin or were untreated, and the group was observed for more than 1 year. None of the individuals in the control groups had received IFN alone or PegIFNα and ribavirin combination treatment. They were treated for a median of 1,395 days (range 75–6,556 days). Fifty-nine of these patients underwent liver biopsy before the treatment and were considered the control group for the propensity-matched study. For the propensity-matched study, 59 patients were selected from the PegIFNα-2a group according to their age, sex, platelet count, and total bilirubin levels, which had been identified as independent pretreatment risk factors for the development of HCC in Study 1. The rates of HCC were analyzed using the Kaplan–Meier method, and the risk of HCC was analyzed particularly in patients with advanced fibrosis of the liver (F3 and F4).
Statistical analysis
Categorical data were compared using the χ2 test or Fisher’s exact test. The distributions of continuous variables were analyzed using Student’s t-test and the Mann–Whitney U-test for two groups. Multivariate analysis was conducted using logistic regression. The cumulative incidence curve was determined using the Kaplan–Meier method and differences between groups were assessed by the log-rank test. For all methods, the level of significance was set at p < 0.05. Multivariate analysis of the risk of HCC was carried out using the Cox proportional hazard model. Statistical analyses were performed using the Statistical Package for the Social Sciences software version 11.0 (SPSS, Chicago, IL, USA). In Study 1, age, sex, platelet count, and total bilirubin levels were identified as independent factors for the development of HCC; therefore, these factors were selected for the propensity-matched control study (Study 2) in which 59 patients from the PegIFNα-2a group were included.
Results
Study 1
We analyzed the factors involved in the development of HCC in patients who received 90 μg PegIFNα-2a weekly or biweekly for more than a year. The incidence of HCC did not differ significantly between the groups treated with PegIFNα-2a weekly and biweekly (34 of 512 vs. 15 of 82, respectively). As shown in Table 2, univariate analysis revealed statistically significant differences in the pretreatment parameters including age, sex, fibrosis of the liver, platelet count, albumin level, and total bilirubin, between patients who developed HCC and those who did not. Endoscopy was carried out in 375 patients, and esophageal varices were noted in 31 of them. The incidence of HCC was higher in patients with esophageal varices than in those without varices [29.0 % (9 of 31) vs. 6.4 % (22 of 344)]. Assessment of on-treatment factors by univariate analysis revealed statistically significant differences in serum ALT, AFP, and HCV RNA levels 24 weeks after the start of PegIFNα-2a maintenance treatment (Table 2).
Multivariate analysis including pretreatment parameters revealed that age, sex, fibrosis of the liver, platelet count, and total bilirubin were independent risk factors for HCC development (Table 3). Multivariate analysis including on-treatment parameters identified ALT levels of ≥41 IU/L and AFP levels of ≥10 ng/L 24 weeks after the start of the PegIFNα-2a therapy as independent risk factors for HCC development (Table 3).
The incidence of HCC was significantly lower in patients with ALT levels of ≤40 IU/L than in those with ALT levels of ≥41 IU/L 24 weeks after the start of observation (Fig. 2). The incidence of HCC was also significantly lower in patients with AFP concentrations of <10 ng/mL at 24 weeks after the start of observation than in those with AFP concentrations of ≥10 ng/mL (Fig. 3). The dose of PegIFNα-2a was reduced to 45 μg in 16 patients because of neutropenia and thrombocytopenia. In addition, PegIFNα-2a was discontinued in 18 patients because of adverse events, including depression (7 patients), interstitial pneumonitis (3 patients), thrombocytopenia (3 patients), neutropenia (1 patient), itching (1 patient), and ascites (3 patients). No statistically significant differences were found between the patients with reduced dosage or treatment interruption and those without treatment modifications with respect to overall survival, HCC incidence, ascites formation, variceal bleeding, hepatic encephalopathy, and 2-point increases in the Child-Pugh score. No patients underwent liver transplantation.
Study 2
We compared the incidence of HCC between 59 patients in the control group and the same number of patients in the PegIFNα-2a group using the matched-pair test. The backgrounds of the patients are shown in Table 4. The PegIFNα-2a group had higher rates of advanced fibrosis (F3 and F4) and active inflammation (A2 and A3). No other differences were found between the two groups, except for the white blood cell count (Table 4).
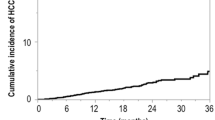
Development of HCC was observed in 2 patients in the PegIFNα-2a group and 8 in the control group. The incidence of HCC was compared between the two groups, using the Kaplan–Meier method. The incidence of HCC in the PegIFNα-2a group was significantly lower than that in the control group (log-rank test, p = 0.0187; Fig. 4). Among the patients with advanced fibrosis of the liver (F3 and F4), those in the PegIFNα-2a group had a lower incidence of HCC than those in the control group. The independent risk factors for the development of HCC were analyzed using the stepwise Cox proportional hazard model. Only PegIFNα-2a administration and age were identified as independent risk factors for the development of HCC (Table 5).
Discussion
The number of HCC cases resulting from HCV infection continues to increase worldwide [19]. To date, IFN therapy is the most effective preventive measure against HCC in patients with chronic hepatitis C; furthermore, the incidence of HCC is reduced in patients who achieve an SVR to IFN [6–9] Therefore, achieving an SVR is the most effective approach for reducing the risk of developing HCC. In Japan, the incidence of HCC is elevated in older patients with hepatitis C. Corroborating this finding, the results of a Japanese study show a higher risk of HCC in patients aged 65 years and more [10]. Therefore, prevention of HCC in aged patients is an important challenge.
In the present multicenter, cooperative, retrospective study conducted in Japan, the incidence of HCC was reduced in patients who received 90 μg PegIFNα-2a weekly or biweekly and had AFP values of <10 ng/mL and ALT values of ≤40 IU/L 24 weeks after the start of the treatment. The results of the matched case–control study of the PegIFNα-2a group and the non-IFN control group show that the incidence of HCC was significantly lower in the PegIFNα-2a group than in the control group, especially in patients with advanced fibrosis of the liver (F3 and F4). However, there could have been a selection bias between the PegIFNα-2a group and the control group (patients who did not agree to receive IFN treatment), because this was a retrospective and non-randomized study. However, concordant with the findings of the HALT-C study [14], the present results show that PegIFNα-2a inhibits the development of HCC in patients with advanced fibrosis of the liver.
Recent studies show that polymorphisms in the host IL28B gene are important factors in the response to PegIFNα and ribavirin combination therapy [20, 21]. However, the mechanism of IL28B involvement in the response to PegIFNα and ribavirin has not been elucidated completely. A recent report has shown that IL28B is a significant factor in the development of HCC as well as in the response to IFN therapy [22]. Further studies are warranted to analyze the relationship between IL28B and inhibition of the development of HCC by PegIFNα in chronic hepatitis C.
Risk factors for the development of HCC have been discussed previously. Increased intrahepatic fat is involved in the development of HCC in chronic hepatitis C patients [23, 24]. In addition, diabetes-associated fat disorder [25, 26], hepatic iron overload [27], advanced fibrosis, older age, and fatty deposits in the liver are risk factors for HCC development [4]. Therefore, it is important to establish strategies to mitigate these risk factors to prevent the development of HCC and thus improve the outcomes of hepatitis C patients.
IFN therapy after HCC treatment is reported to inhibit the recurrence of tumors [28, 29], and a meta-analysis has revealed a trend toward inhibition of the recurrence of HCC [30, 31]. The prevention of HCC is an important issue that needs to be addressed to improve the survival of chronic hepatitis C patients. The findings of the present study and the HALT-C trial [14] indicate the effectiveness of long-term administration of maintenance IFN for preventing the development of HCC in chronic hepatitis C patients without an SVR. Improvement in ALT levels is also known to be an important predictor for the prevention of HCC [32]. A low AFP value during IFN administration is also recognized as a significant indicator of a lower risk of HCC [33, 34]. Recently, Osaki et al. [35] reported that a decrease of serum AFP during treatment with IFN was associated with a reduced incidence of HCC. Taking these findings and our own together, we conclude that maintenance administration of low-dose PegIFNα-2a weekly or biweekly to non-SVR patients with chronic hepatitis C decreases the incidence of HCC, especially in patients whose serum ALT and AFP levels are within the normal range 24 weeks after the start of treatment. The preventive effects of IFN against the development of HCC without elimination of the virus may be associated with its anti-carcinogenic effects [16, 35]; however, the precise mechanism should be investigated.
The limitations of the present study are that it is retrospective and multicentric; therefore, potentially there may have been a selection bias. However, the reduction of the rate of development of HCC by maintenance administration of PegIFNα-2a in the patients in whom serum ALT and AFP levels were within the normal ranges 24 weeks after the start of treatment may be attributable to the anticarcinogenic effects of IFN without elimination of the virus.
Conclusion
The incidence of HCC was lower in non-SVR patients with chronic hepatitis C who were administered with maintenance low-dose PegIFNα-2a; especially in those whose serum ALT and AFP levels were within the normal ranges 24 weeks after the start of treatment.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi:10.3322/canjclin.55.2.74.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi:10.1016/S0140-6736(03)14964-1.
Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano K, et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671–5. doi:10.1002/hep.1840120409.
Namiki I, Nishiguchi S, Hino K, Suzuki F, Kumada H, Itoh T, et al. Management of hepatitis C; Report of the consensus meeting at the 45th annual meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:347–68. doi:10.1111/j.1872-034X.2010.00642.x.
Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shin JW, Gojobori T, et al. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002;99:11584–9. doi:10.1073/pnas.242608099.
Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, et al. Effect of interferon therapy on hepatocellular carcinoma in patients with chronic hepatitis type C: a long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124–30.
Imai Y, Kawata S, Tamura S, Yabuuchi I, Noda S, Inada M, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Ann Intern Med. 1998;129:94–9.
Bruno S, Stroffolini T, Colombo M, Bollani S, Benveguu L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87. doi:10.1002/hep.21492.
Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virological response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–84.
Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518–27. doi:10.1002/hep.23691.
Amarapurkar D, Han KH, Chan HL, Ueno Y, Asia-Pacific working party on prevention of hepatocellular carcinoma. Application of surveillance programs for hepatocellular carcinoma in the Asia-Pacific Region. J Gastroenterol Hepatol. 2009;24:955–61. doi:10.1111/j.1440-1746.2009.05805.x.
Tamura Y, Yamagiwa S, Aoki Y, Kurita S, Suda T, Ohkoshi S, et al. Serum alpha-fetoprotein levels during and after interferon therapy and the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci. 2009;54:2530–7.
Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–41. doi:10.1056/NEJMoa0707615.
Lok AS, Everhart JE, Wright EC, Di Bischeglie AM, Kim HY, Stering RK, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–9. doi:10.1053/j.gastro.2010.11.050.
Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990–9. doi:10.1053/j.gastro.2010.11.050.
Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, et al. Prolonged-interferon therapy reduces hepatocarcinogenesis in aged-patients with chronic hepatitis C. J Med Virol. 2007;79:1095–102. doi:10.1002/jmv.20866.
Poynard T, Moussali J, Ratziu V, Regimberu C, Opolan P. Effects of interferon therapy in “non-responder” patients with chronic hepatitis C. J Hepatol. 1999;31S:178–83. doi:10.1016/S0168-8278(99)80397-3.
Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer P. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. doi:10.1016/0270-9139(94)90250-X, doi:10.1002/hep.1840190629.
Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–8. doi:10.1053/j.gastro.2010.12.032.
Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi:10.1038/nature08309.
Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nature. 2009;41:1105–9.
Fabris C, Falleti E, Cussigh A, Bitetto D, Fontanini E, Bignulin S, et al. IL-28B rs 12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol. 2011;54:716–22. doi:10.1016/j.jhep.2010.07.019.
Kurosaki M, Hosokawa T, Matsunaga K, Hirayama I, Tanaka T, Sato M, et al. Hepatic steatosis in chronic hepatitis C is a significant risk factor for developing hepatocellular carcinoma independent of age, sex, obesity, fibrosis stage and response to interferon therapy. Hepatol Res. 2010;40:870–7. doi:10.1111/j.1872-034X.2010.00692.x.
Koike K. Steatosis, liver injury, and hepatocarcinogenesis in hepatitis C viral infection. J Gastroenterol. 2009;44(Suppl 19):82–8. doi:10.1007/s00535-008-2276-4.
Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofman WP, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856–62. doi:10.1002/hep.22251.
Lai MS, Hsieh MS, Chiu YH, Chen TH. Type 2 diabetes and hepatocellular carcinoma: a cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43:1295–302. doi:10.1002/hep.21208.
Furutani T, Hino K, Okuda M, Gondo T, Nishina S, Kitase A, et al. Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2006;130:2087–98. doi:10.1053/j.gastro.2006.02.060.
Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418–22. doi:10.1046/j.0007-1323.2001.02054.x.
Kudo M, Sakaguchi Y, Chung H, Hatanaka K, Hagiwara S, Ishikawa E, et al. Long-term interferon maintenance therapy improves survival in patients with HCV-related hepatocellular carcinoma after curative radiofrequency ablation. A matched case–control study. Oncology. 2007;72(Suppl 1):132–8. doi:10.1159/000111719.
Singal AK, Freeman DH Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851–8. doi:10.1111/j.1365-2036.2010.04414.x.
Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17:287–92. doi:10.1111/j.1365-2893.2009.01181.x.
Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, et al. Interferon-induced prolonged biochemical response reduces hepatocarcinogenesis in hepatitis C virus infection. J Med Virol. 2007;79:1485–90. doi:10.1002/jmv.20925.
Nomura H, Kashiwagi Y, Hirano R, Tanimoto H, Tsutsumi N, Higashi M, et al. Efficacy of low dose long-term interferon monotherapy in aged patients with chronic hepatitis C genotype 1 and its relation to alpha-fetoprotein: a pilot study. Hepatol Res. 2007;37:490–7. doi:10.1111/j.1872-034X.2007.00073.x.
Chen TM, Huang PT, Tsai MH, Lin LF, Liu CC, Ho KS, et al. Predictors of alpha-fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a-ribavirin combination therapy. J Gastroenterol Hepatol. 2007;22:669–75. doi:10.1111/j.1440-1746.2007.04898.x.
Osaki Y, Ueda Y, Marusawa H, Nakajima J, Kimura T, Kita R, et al. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol. 2012;47:444–51.
Acknowledgments
This study was supported by a Grant-in-Aid from the Japanese Ministry of Health, Welfare, and Labor.
Conflict of interest
Namiki Izumi received lecture fees from Chugai Co. and MSD Co. in 2011.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Izumi, N., Asahina, Y., Kurosaki, M. et al. Inhibition of hepatocellular carcinoma by PegIFNα-2a in patients with chronic hepatitis C: a nationwide multicenter cooperative study. J Gastroenterol 48, 382–390 (2013). https://doi.org/10.1007/s00535-012-0641-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0641-9