Abstract
Background
Des-γ-carboxy prothrombin (DCP) is an established tumor marker for hepatocellular carcinoma (HCC), but the precise mechanism of its production remains unknown. We have recently demonstrated that cytoskeletal rearrangement during the phenotypic changes involved in epithelial mesenchymal transition (EMT) plays a crucial role in DCP production through the impairment of vitamin K uptake. However, DCP production in long-lasting severe hypoxic conditions with nutrient deprivation–such as transarterial embolization–remains unknown.
Methods
We examined the effects of long-lasting hypoxia with nutrient deprivation, as well as the constitutive expression of hypoxia-inducible factor (HIF)-1α, on EMT status, DCP production, and protein synthesis in human hepatoma cell lines by enzyme-linked immunosorbent assay, immunofluorescent studies, and western blotting. Immunohistochemistry findings for DCP, anti-hepatocyte paraffin 1 (Hep Par 1), and vimentin were examined using human resected HCC samples.
Results
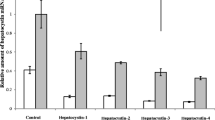
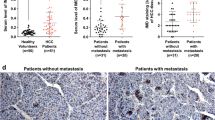
Both severe hypoxia with nutrient deprivation and HIF-1α transfection led to the cessation of DCP production, by attenuating protein synthesis through the hypophosphorylation of mammalian target of rapamycin and its effector proteins, indicative of a further phenotypic shift involving impaired liver-specific protein synthesis. In immunohistochemistry, the distribution of DCP- and Hep Par 1-positive HCC cells was mostly similar and vimentin-positive HCC cells were frequently observed in the areas that were negative for Hep Par 1 and/or DCP.
Conclusions
HCC cells produce DCP when they undergo mild phenotypic changes. However, when HCC cells adopt mesenchymal properties they lose their capacity for protein synthesis, and the production of DCP is attenuated. Building upon our previous works, it appears that DCP could be a unique tumor marker that reflects the stepwise phenotypic changes of HCC.





Similar content being viewed by others
References
Liebman H, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, et al. Des-γ-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427–31.
Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, et al. Des-gamma-carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37:1114–21.
Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, et al. Des-γ-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–8.
Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma. Hepatol Res. 2008;38:37–51.
Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systemic review. Hepatol Int. 2008;2:17–30.
Leerapun A, Suravarapu SV, Bida JP, Clark RJ, Sanders EL, Mettler TA, et al. The utility of AFP-L3% in the diagnosis of hepatocellular carcinoma: evaluation in a U.S. referral population. Clin Gastroenterol Hepatol. 2007;5:394–402.
Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, et al. Des-γ-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–9.
Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, HALT-C Trial Group, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502.
Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16.
Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26:319–31.
Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–9.
Christofori G. New signals from the invasive front. Nature. 2006;441:444–50.
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4.
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol. 2008;10:295–305.
Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–43.
Luo Y, He DL, Ning L, Shen SL, Li L, Li X, et al. Over-expression of hypoxia-inducible factor-1α increases the invasive potency of LNCap cells in vitro. BJU Int. 2006;98:1315–9.
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Over expression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5.
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–49.
Murata K, Sakamoto A. Impairment of clathrin-mediated endocytosis via cytoskeletal change by epithelial to fibroblastoid conversion in HepG2 cells: a possible mechanism of des-γ-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol. 2008;33:1149–55.
Murata K, Suzuki H, Okano H, Oyamada T, Yasuda Y, Sakamoto A. Cytoskeletal changes during epithelial-to-fibroblastoid conversion as a crucial mechanism of des-γ-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol. 2009;35:1005–14.
Murata K, Suzuki H, Okano H, Oyamada T, Yasuda Y, Sakamoto A. Hypoxia-induced des-γ-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol. 2010;36:161–70.
Mizuta T, Ozaki I, Eguchi Y, Yasutake T, Kawazoe S, Fujimoto K, et al. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment. Cancer. 2006;106:867–72.
Shimada M, Yonemura Y, Ijichi H, Harada N, Shiotani S, Ninomiya M, et al. Living donor liver transplantation for hepatocellular carcinoma: a special reference to a preoperative des-gamma-carboxy prothrombin value. Transplant Proc. 2005;37:1177–9.
Arsham AM, Howell JJ, Simons MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–60.
Pouyssegur J, Dayan F, Mazure NM. Hypoxia signaling in cancer and approaches to enforce tumor regression. Nature. 2006;441:437–43.
Wouters BG, Koritzinsky M. Hypoxia signaling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64.
Boutilier RG, St-Pierre J. Surviving hypoxia without really dying. Comp Biochem Physiol A Mol Integr Physiol. 2000;126:481–90.
Bristow RG, Hill RP. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–92.
Gulhati P, Bowen KA, Liu J, et al. mTORC1 and mTORC2 regulate EMT, motility and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56.
Butler SL, Dong H, Cardona D, Jia M, Zheng R, Zhu H, et al. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest. 2008;88:78–88.
Kumagai I, Masuda T, Sato S, Ishikawa K. Immunoreactivity to monoclonal antibody, Hep Par 1, in human hepatocellular carcinomas according to histological grade and histological pattern. Hepatol Res. 2001;20:312–9.
Sato T, Tateishi R, Yoshida H, Ohki T, Masuzaki R, Imamura J, et al. Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol Int. 2009;3:544–50.
Takahashi S, Kudo M, Chung H, Inoue T, Ishikawa E, Kitai S, et al. PIVKA-II is the best prognostic predictor in patients with hepatocellular carcinoma after radiofrequency ablation therapy. Oncology. 2008;75:91–8.
Ueshima K, Kudo M. PIVKA-II is a predictive marker in the treatment response of sorafenib to hepatocellular carcinoma. Kanzo. 2010;51:681–3. (in Japanese).
Acknowledgments
This work was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, H., Murata, K., Gotoh, T. et al. Phenotype-dependent production of des-γ-carboxy prothrombin in hepatocellular carcinoma. J Gastroenterol 46, 1219–1229 (2011). https://doi.org/10.1007/s00535-011-0432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0432-8