Abstract
Background
The induction of intestinal trefoil factor (ITF) has been reported to depend on hypoxia-inducible factor-1 (HIF-1). Nitric oxide modulates HIF-1 activity. The present study aims to analyze the role of nitric oxide in jejunum damage induced by indomethacin and its ability to modulate epithelial function through the expression of ITF.
Methods
Rats received indomethacin (7.5 mg/kg, s.c., twice), and a time course analysis of damage was performed (24–96 h after the first administration). In these animals, the role of nitric oxide was analyzed by using 1400W, a selective iNOS activity inhibitor (5 mg/kg, i.p./day), on: (1) intestinal damage, (2) ulcer healing, (3) the presence of nitrated proteins in the jejunum and (4) the protein expression of inducible nitric oxide synthase (iNOS), HIF-1α and ITF.
Results
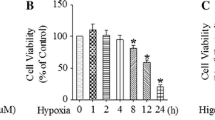
Indomethacin induced damage in the jejunum that was apparent at 24 h and peaked at 48–72 h. An increase in iNOS, HIF-1α, ITF and nitrated proteins was observed in the injured jejunum. Immunoprecipitation of HIF-1α allowed determination of the nitration/nitrosylation of this protein by using nitrotyrosine and nitrocysteine antibodies. Blockade of iNOS activity did not significantly modify damage or iNOS expression, but did significantly impede ITF induction, HIF-1α stabilization and HIF-1α detection with antibodies against nitrated proteins. In parallel to these results, pre-treatment with 1400W delayed the healing of the ulcer provoked by indomethacin.
Conclusions
These results suggest that iNOS-derived NO is involved in HIF-1α stabilization, probably through S-nitrosylation, and ITF expression in goblet cells of the damaged jejunum of indomethacin-treated rats and mediates ulcer healing.







Similar content being viewed by others
References
Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–83.
Playford RJ, Marchbank T, Chinery R, Evison R, Pignatelli M, Boulton RA, et al. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995;108:108–16.
Kato K, Chen MC, Nguyen M, Lehmann FS, Podolsky DK, Soll AH. Effects of growth factors and trefoil peptides on migration and replication in primary oxyntic cultures. Am J Physiol. 1999;276:G1105–16.
Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932–8.
Kjellev S. The trefoil factor family––small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66:1350–69.
Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800.
Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–34.
Hernandez C, Santamatilde E, McCreath KJ, Cervera AM, Diez I, Ortiz-Masia D, et al. Induction of trefoil factor (TFF)1, TFF2 and TFF3 by hypoxia is mediated by hypoxia inducible factor-1: implications for gastric mucosal healing. Br J Pharmacol. 2009;156:262–72.
Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–23.
Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270:781–90.
Sandau KB, Fandrey J, Brune B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97:1009–15.
Sogawa K, Numayama-Tsuruta K, Ema M, Abe M, Abe H, Fujii-Kuriyama Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc Natl Acad Sci USA. 1998;95:7368–73.
Yin JH, Yang DI, Ku G, Hsu CY. iNOS expression inhibits hypoxia-inducible factor-1 activity. Biochem Biophys Res Commun. 2000;279:30–4.
Agani FH, Puchowicz M, Chavez JC, Pichiule P, LaManna J. Role of nitric oxide in the regulation of HIF-1alpha expression during hypoxia. Am J Physiol Cell Physiol. 2002;283:C178–86.
Bove PF, Hristova M, Wesley UV, Olson N, Lounsbury KM, van der Vliet A. Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor-1 alpha. J Biol Chem. 2008;283(26):17919–28.
Mateo J, Garcia-Lecea M, Cadenas S, Hernandez C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–44.
Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74.
Ito M, Tanaka S, Kim S, Kuwai T, Matsutani N, Kamada T, et al. The specific expression of hypoxia inducible factor-1alpha in human gastric mucosa induced by nonsteroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2003;18(Suppl 1):90–8.
Ortiz-Masia D, Hernandez C, Quintana E, Velazquez M, Cebrian S, Riano A, et al. iNOS-derived nitric oxide mediates the increase in TFF2 expression associated with gastric damage: role of HIF-1. FASEB J. 2010;24:136–45.
Kane S, Lu F, Kornbluth A, Awais D, Higgins PD. Controversies in mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2009;15:796–800.
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–6.
Souza MH, Lemos HP, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53:791–6.
Akiba Y, Nakamura M, Mori M, Suzuki H, Oda M, Kimura H, et al. Inhibition of inducible nitric oxide synthase delays gastric ulcer healing in the rat. J Clin Gastroenterol. 1998;27(Suppl 1):S64–73.
Takeuchi K, Hatazawa R, Tanigami M, Tanaka A, Ohno R, Yokota A. Role of endogenous nitric oxide (NO) and NO synthases in healing of indomethacin-induced intestinal ulcers in rats. Life Sci. 2007;80:329–36.
Parasher G, Frenklakh L, Goodman, Siddiqui T, Nandi J, Levine RA. Nitric oxide inhibitors ameliorate indomethacin-induced enteropathy in rats. Dig Dis Sci. 2001;46:2536–41.
Evans SM, Laszlo F, Whittle BJ. Site-specific lesion formation, inflammation and inducible nitric oxide synthase expression by indomethacin in the rat intestine. Eur J Pharmacol. 2000;388:281–5.
Nishida K, Ohta Y, Ishiguro I. Relation of inducible nitric oxide synthase activity to lipid peroxidation and nonprotein sulfhydryl oxidation in the development of stress-induced gastric mucosal lesions in rats. Nitric Oxide. 1998;2:215–23.
Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–81.
Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275–82.
Mylonis I, Chachami G, Samiotaki M, Panayotou G, Paraskeva E, Kalousi A, et al. Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1alpha. J Biol Chem. 2006;281:33095–106.
Diez I, Calatayud S, Hernandez C, Quintana E, O’Connor JE, Esplugues JV, et al. Nitric oxide, derived from inducible nitric oxide synthase, decreases hypoxia inducible factor-1alpha in macrophages during aspirin-induced mesenteric inflammation. Br J Pharmacol. 2010;159(8):1636–45.
Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–71.
Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–31.
Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–32.
Anand RJ, Gribar SC, Li J, Kohler JW, Branca MF, Dubowski T, et al. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1alpha-dependent manner. J Leukoc Biol. 2007;82:1257–65.
Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–27.
Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, et al. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–97.
Acknowledgments
We thank Dr. Podolsky for providing us with the ITF antibody. This study was funded by grants SAF2007-064201 from the Ministerio de Educación y Cultura, CIBERehd CB06/04/0071 and PI081325, from the Ministerio de Sanidad y Consumo, and ACOMP07-297 and PROMETEO/2010/060 from Generalitat Valenciana.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Riaño and D. Ortiz-Masià contributed equally to this work.
Rights and permissions
About this article
Cite this article
Riaño, A., Ortiz-Masià, D., Velázquez, M. et al. Nitric oxide induces HIF-1α stabilization and expression of intestinal trefoil factor in the damaged rat jejunum and modulates ulcer healing. J Gastroenterol 46, 565–576 (2011). https://doi.org/10.1007/s00535-011-0374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0374-1