Abstract
Background
To distinguish malignant from benign branch duct (BD)-intraductal papillary mucinous neoplasm (IPMN) still remains difficult. Recently, we revealed that MSX2 was frequently expressed in pancreatic cancer and its expression was correlated with aggressive behavior of the cancer. The aim of this study was to assess the involvement of MSX2 in IPMN development and whether its expression would differentiate malignant from benign IPMN.
Methods
Seventeen microdissected lesions and 45 IPMN tissues were used for quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) and immunohistochemistry, respectively. The role of MSX2 in the pancreatic duct cell was assessed by the induced expression of MSX2 in a normal human pancreatic duct epithelial cell line (HPDE).
Results
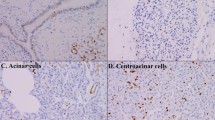
Malignant IPMN expressed significantly higher levels of MSX2 mRNA than benign IPMN lesions. MSX2 protein expression was frequently found in borderline and malignant lesions (20/29, 68.9%), while its expression was seen in only one of 16 benign IPMN tissues. Univariate analysis showed that nodules of 6 mm or more and MSX2 expression were significantly correlated with the malignancy of BD-IPMN (P = 0.022 and 0.0026, respectively), and multivariate analysis revealed that only MSX2 expression was identified as an independent factor to predict malignant BD-IPMN. HPDE cells expressing MSX2 showed increased cellular proliferation compared to control cells.
Conclusions
Based on our results, MSX2 plays a pivotal role in the development of IPMN through growth stimulation of tumor cells, and its expression was identified as an independent predictive factor for malignancy of BD-IPMN.



Similar content being viewed by others
Abbreviations
- IPMN:
-
Intraductal papillary-mucinous tumor of the pancreas
- QRT-PCR:
-
Quantitative real-time reverse transcription-polymerase chain reaction
- IPMA:
-
Intraductal papillary-mucinous adenoma
- IPMB:
-
Borderline intraductal papillary-mucinous neoplasm
- IPMC:
-
Intraductal papillary-mucinous carcinoma
- IC-IPMN:
-
Invasive carcinoma derived from intraductal papillary-mucinous carcinoma
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- PBS:
-
Phosphate-buffered saline
References
Loftus JEV, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Gastroenterology. 1996;110:1909–18.
Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–7.
Hara T, Yamaguchi T, Ishihara T, Tsuyuguchi T, Kondo F, Kato K, et al. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34–43.
Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–6.
Doi R, Fujimoto K, Wada M, Imamura M. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery. 2002;132:80–5.
Matsumoto T, Aramaki M, Yada K, Hirano S, Himeno Y, Shibata K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–5.
Kitagawa Y, Unger TA, Taylor S, Kozarek RA, Traverso LW. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12–8. discussion 18–19.
Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–9.
Satoh K, Sawai T, Shimosegawa T, Koizumi M, Yamazaki T, Mochizuki F, et al. The point mutation of c-Ki-ras at codon 12 in carcinoma of the pancreatic head region and in intraductal mucin-hypersecreting neoplasm of the pancreas. Int J Pancreatol. 1993;14:135–43.
Satoh K, Shimosegawa T, Moriizumi S, Koizumi M, Toyota T. K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas. 1996;12:362–8.
Satoh K, Kanno A, Hamada S, Hirota M, Umino J, Masamune A, et al. Expression of Sonic hedgehog signaling pathway correlates with the tumorigenesis of intraductal papillary mucinous neoplasm of the pancreas. Oncol Rep. 2008;19:1185–90.
Satoh K, Sasano H, Shimosegawa T, Koizumi M, Yamazaki T, Mochizuki F, et al. An immunohistochemical study of the c-erbB-2 oncogene product in intraductal mucin-hypersecreting neoplasms and in ductal cell carcinomas of the pancreas. Cancer. 1993;72:51–6.
Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271–8.
Fukushige S, Furukawa T, Satoh K, Sunamura M, Kobari M, Koizumi M, et al. Loss of chromosome 18q is an early event in pancreatic ductal tumorigenesis. Cancer Res. 1998;58:4222–6.
Sugiyama M, Atomi Y, Saito M. Intraductal papillary tumors of the pancreas: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1998;48:164–71.
Yasuda H, Takada T, Amano H, Yoshida M. Surgery for mucin-producing pancreatic tumor. Hepatogastroenterology. 1998;45:2009–15.
Lee SY, Lee KT, Lee JK, Jeon YH, Choi D, Lim JH, et al. Long-term follow up results of intraductal papillary mucinous tumors of pancreas. J Gastroenterol Hepatol. 2005;20:1379–84.
Traverso LW. Surgical treatment of intraductal papillary mucinous neoplasms of the pancreas: the aggressive approach. J Gastrointest Surg. 2002;6:662–3.
Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, et al. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274–8.
Irie H, Yoshimitsu K, Aibe H, Tajima T, Nishie A, Nakayama T, et al. Natural history of pancreatic intraductal papillary mucinous tumor of branch duct type: follow-up study by magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. 2004;28:117–22.
Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Takasawa O, et al. Mode of progression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005;40:744–51.
Tanaka M. International consensus guidelines for the management of IPMN and MCN of the pancreas. Nippon Shokakibyo Gakkai Zasshi. 2007;104:1338–43.
Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–64.
Takahashi Y, Le Douarin N. cDNA cloning of a quail homeobox gene and its expression in neural crest-derived mesenchyme and lateral plate mesoderm. Proc Natl Acad Sci USA. 1990;87:7482–6.
Davidson DR, Crawley A, Hill RE, Tickle C. Position-dependent expression of two related homeobox genes in developing vertebrate limbs. Nature. 1991;352:429–31.
Jowett AK, Vainio S, Ferguson MW, Sharpe PT, Thesleff I. Epithelial-mesenchymal interactions are required for msx 1 and msx 2 gene expression in the developing murine molar tooth. Development. 1993;117:461–70.
Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, et al. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–37.
Suzuki M, Tanaka M, Iwase T, Naito Y, Sugimura H, Kino I. Over-expression of HOX-8, the human homologue of the mouse Hox-8 homeobox gene, in human tumors. Biochem Biophys Res Commun. 1993;194:187–93.
Hamada S, Satoh K, Hirota M, Kimura K, Kanno A, Masamune A, et al. Bone morphogenetic protein 4 induces epithelial–mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J Cell Physiol. 2007;213:768–74.
Satoh K, Hamada S, Kimura K, Kanno A, Hirota M, Umino J, et al. Up-regulation of MSX2 enhances the malignant phenotype and is associated with twist 1 expression in human pancreatic cancer cells. Am J Pathol. 2008;172:926–39.
Longnecker DS, Adler G, Hruban RH, Kloppel G. Intraductal papillary mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. Pathology and genetics. Tumours of the digestive system. WHO classification of tumours. Lyon: IARC Press; 2000. p. 237–40.
Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148:1763–70.
Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 2001;158:419–29.
Kim J, Reber HA, Hines OJ, Kazanjian KK, Tran A, Ye X, et al. The clinical significance of MAGEA3 expression in pancreatic cancer. Int J Cancer. 2006;118:2269–75.
Ohuchida K, Mizumoto K, Fujita H, Yamaguchi H, Konomi H, Nagai E, et al. Sonic hedgehog is an early developmental marker of intraductal papillary mucinous neoplasms: clinical implications of mRNA levels in pancreatic juice. J Pathol. 2006;210:42–8.
Takahashi C, Akiyama N, Matsuzaki T, Takai S, Kitayama H, Noda M. Characterization of a human MSX-2 cDNA and its fragment isolated as a transformation suppressor gene against v-Ki-ras oncogene. Oncogene. 1996;12:2137–46.
Dodig M, Tadic T, Kronenberg MS, Dacic S, Liu YH, Maxson R, et al. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev Biol. 1999;209:298–307.
Liu YH, Tang Z, Kundu RK, Wu L, Luo W, Zhu D, et al. Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev Biol. 1999;205:260–74.
Satoh K, Ginsburg E, Vonderhaar BK. Msx-1 and Msx-2 in mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:195–205.
Satoh K, Hovey RC, Malewski T, Warri A, Goldhar AS, Ginsburg E, et al. Progesterone enhances branching morphogenesis in the mouse mammary gland by increased expression of Msx2. Oncogene. 2007;26:7526–34.
Brody JR, Witkiewicz A, Williams TK, Kadkol SS, Cozzitorto J, Durkan B, et al. Reduction of pp32 expression in poorly differentiated pancreatic ductal adenocarcinomas and intraductal papillary mucinous neoplasms with moderate dysplasia. Mod Pathol. 2007;20:1238–44.
Bai J, Brody JR, Kadkol SS, Pasternack GR. Tumor suppression and potentiation by manipulation of pp32 expression. Oncogene. 2001;20:2153–60.
Biankin AV, Biankin SA, Kench JG, Morey AL, Lee CS, Head DR, et al. Aberrant p16 (INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861–8.
Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323–4.
Acknowledgments
We thank Dr. M.S. Tsao for providing HPDE cells. This work was supported in part by Grants-in-Aid #21590870 and #20390202 from the Ministry of Education, Science, Sports and Culture in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, K., Hamada, S., Kanno, A. et al. Expression of MSX2 predicts malignancy of branch duct intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol 45, 763–770 (2010). https://doi.org/10.1007/s00535-010-0200-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0200-1