Abstract
Background
We conducted a randomized controlled trial (RCT) to evaluate the clinical efficacy of an intravenous fluoroquinolone, ciprofloxacin (CIP), in patients with biliary tract infection requiring biliary drainage using imipenem/cilastatin (IPM/CS) as a control.
Methods
After the initial collection of bile, patients were randomly assigned to receive CIP at 300 mg twice daily by intravenous drip infusion or IPM/CS at 500 mg twice daily by intravenous drip infusion with the envelope method.
Results
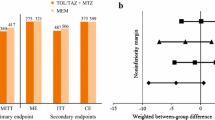
The characteristics of the 104 patients evaluated for efficacy were well balanced. The clinical response rates were 100.0% (50/50 patients) in the CIP group and 94.4% (51/54) in the IPM/CS group. The difference in clinical response rate between groups (CIP, IPM/CS) was 5.56% (90% confidence interval: −0.26%, 13.95%), and the non-inferiority of CIP to IPM/CS was confirmed. Adverse events for which causal relationships with the study drugs could not be ruled out developed in 5.4% (3/56) of patients in the CIP group and 5.2% (3/58) of patients in the IPM/CS group, and none of them were serious.
Conclusions
The clinical efficacy of CIP in treating biliary tract infection requiring drainage was comparable to that of IPM/CS. These findings suggest that CIP is useful as a new therapeutic option for biliary tract infection.






Similar content being viewed by others
Notes
Package insert of Tienam (imipenem/cilastatin).
References
Tazuma S, Iwamoto K, Hyogo H, Shimatani T, Ono H, Hiyama E, et al. Clinical experience of injectable ciprofloxacin (CIP) for biliary tract infection. J Biliary Tract Pancreas. 2003;24:781–5.
Sung JJ, Lyon DJ, Suen R, Chung SC, Co AL, Cheng AF, et al. Intravenous ciprofloxacin as treatment for patients with acute suppurative cholangitis: randomized, controlled clinical trial. J Antimicrob Chemother. 1995;35:855–64.
Elkhajli H, Kamili N, Linger L, Levêque D, Pompei D, Monteil H, et al. In vitro time-kill curves of cefepime and cefpirome combined with amikacin, gentamicin or ciprofloxacin against Klebsiella pneumoniae producing extended-spectrum beta-lactamase. Chemotherapy. 1997;43:245–53.
Hirakata Y, Izumikawa K, Yamaguchi T, Takemura H, Tanaka H, Yoshida R, et al. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1998;42:2006–11.
Hara K, Kohno S, Kadota J, Tomono K, Hirakata Y, Maesaki S, et al. Clinical evaluation of injectable ciprofloxacin for bacterial pneumonia–Ceftazidime-controlled phase-III clinical study. Chemotherapy. 1997;45:901–22. (in Japanese with English abstract).
Kobayashi H, Kawai H, Oshitani H, Koike T, Sakayori S, Ohnishi K, et al. Late phase-II clinical study of injectable ciprofloxacin. Chemotherapy. 1997;45:846–71. (in Japanese with English abstract).
Aikawa N, Sasaki J, Iwai S, Kunimatsu M, Furuhata H, Watanabe T, et al. Clinical evaluation of injectable ciprofloxacin for severe or refractory infection in surgical/gynecological areas. Chemotherapy. 1997;45:936–50. (in Japanese with English abstract).
Endo S, Inoue Y, Kikuchi M, Yamada Y, Nakae H. Clinical efficacy of ciprofloxacin for injection (ciprofloxacin injection) in emergency medical care area. Antibiot Chemother. 2001;17:980–6. (in Japanese with English abstract).
Ichiyama S, Mori T, Yamaguchi K, Hayashi M, Yamanaka K, Kurokawa Y, et al. Surveillance of susceptibility of clinical isolates from severe infection to various antimicrobials. Jpn J Antibiot. 2001;54:401–47. (in Japanese with English abstract).
Parry MF, Smego DA, Digiovanni MA. Hepatobiliary kinetics and excretion of ciprofloxacin. Antimicrob Agents Chemother. 1998;32:982–5.
Tanimura H, Ishihara H, Kobayashi N, Tsuchiya Y, Uchiyama K. National survey report for gallstones for fiscal 1997. J Jpn Biliary Assoc. 1998;12:276–93. (in Japanese with English abstract).
Takesue Y, Nakajima K, Ichiki K, Wada Y, Kuramoto M, Yanagi H, et al. Usage of antimicrobials for biliary tract infection in Europe and the United States. J Jpn Soc Surg Infect. 2006;3:227–32. (in Japanese with English abstract).
Gerecht WB, Henry NK, Hoffman WW, Muller SM, LaRusso NF, Rosenblatt JE. Prospective randomized comparison of mezlocillin therapy alone with combined ampicillin and gentamicin therapy for patients with cholangitis. Arch Intern Med. 1989;149:1279–84.
Muller EL, Pitt HA, Thompson JE Jr, Doty JE, Mann LL, Manchester B. Antibiotics in infections of the biliary tract. Surg Gynecol Obstet. 1987;165:285–92.
Thompson JE Jr, Pitt HA, Doty JE, Coleman J, Irving C. Broad spectrum penicillin as an adequate therapy for acute cholangitis. Surg Gynecol Obstet. 1990;171:275–82.
Thompson JE Jr, Bennion RS, Roettger R, Lally KP, Hopkins JA, Wilson SE. Cefepime for infections of the biliary tract. Surg Gynecol Obstet. 1993;177(Suppl):30–4.
Chacon JP, Criscuolo PD, Kobata CM, Ferraro JR, Saad SS, Reis C. Prospective randomized comparison of pefloxacin and ampicillin plus gentamicin in the treatment of bacteriologically proven biliary tract infections. J Antimicrob Chemother. 1990;26(Suppl B):167–72.
Committee on Guidelines for the Management of Acute Cholangitis. Guidelines for the Management of Acute Cholangitis/Cholecystitis 1st ed. Tokyo: Igaku Tosho Shuppan, 2005.
Raymond DP, Pelletier SJ, Crabtree TD, Gleason TG, Hamm LL, Pruett TL, et al. Impact of a rotating empiric antibiotic schedule on infectious mortality in an intensive care unit. Crit Care Med. 2001;29:1101–8.
Acknowledgments
We express our deepest appreciation to the members of the BTI Therapy Research Group and to their institutions. For full details, please see the Appendix.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
See the Appendix for full details of the BTI Therapy Research Group.
Appendix
Appendix
The Biliary Tract Infection (BTI) Therapy Research Group consists of the following physicians: Susumu Tazuma, Department of General Medicine, Hiroshima University Graduate School of Medical Science, Programs of Applied Medicine, Clinical Pharmacotherapy; Yoshinori Igarashi, Division of Gastroenterology and Hepatology, Department of Internal Medicine of Toho University Omori Medical Center; Hirotaka Ohara, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences; Yoshinari Furukawa and Yukio Kuwata, Department of Gastroenterology, Hiroshima Red Cross Hospital & Atomic-Bomb Survivors Hospital; Hironori Tokumou, Yasumasa Asamoto, and Kei Shinagawa, Department of Gastroenterology, Hiroshima General Hospital; Keiji Hanada, Naomichi Hirano, Tomohiro Iiboshi, Yasutaka Ishii, and Fumiaki Hino, Department of Gastroenterology, Onomichi General Hospital; Hitoshi Yokoya and Souichirou Yamasaki, Department of Internal Medicine, Fuchu City Fuchu Kita Hospital; Minoru Sakomoto, Department of Gastroenterology, National Hospital Organaization Kure Medical Center; Yoshiaki Asami and Hiroyuki Ochikubo, Kajikawa Hospital; Toshihide Ohya, Yoshio Kuga, Daizaburou Hirata, Yoshito Takemura, and Aki Kogame, Department of Gastroenterology, Chugoku Rousai Hospital; Toshio Tsuyuguchi, Yuji Sakai, and Shin Tsuchiya, Department of Medicine and Clinical Oncology, Graduate School of Medicine, Chiba University; Yasuharu Kikuchi, Department of Gastroenterology, Numazu City Hospital; Takeshi Nihei, Department of Gastroenterology, Mito Saiseikai General Hospital; Hidekazu Kurata, Department of Gastroenterology, Shimotsuga General Hospital; Yoshimi Ishii, Department of Internal Medicine, Chiba Cardiovascular Center; Kazuo Inui, Kazumu Okushima, Hironao Miyoshi, Yuta Nakamura, and Masashi Hattori, Department of Internal Medicine, Second Teaching Hospital, Fujita Health University School of Medicine.
Rights and permissions
About this article
Cite this article
Tazuma, S., Igarashi, Y., Tsuyuguchi, T. et al. Clinical efficacy of intravenous ciprofloxacin in patients with biliary tract infection: a randomized controlled trial with carbapenem as comparator. J Gastroenterol 44, 781–792 (2009). https://doi.org/10.1007/s00535-009-0067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0067-1