Abstract
Background
The rates of sustained virologic response (SVR) and relapse with pegylated interferon alpha 2b (peginterferon) plus ribavirin in patients with genotype-1 chronic hepatitis C (CHC) are approximately 50 and 30%, respectively. We investigated whether SVR and transient response (TR) can be differentiated during treatment using new indices calculated from early viral kinetics and the timing of when hepatitis C virus (HCV)-RNA becomes undetectable.
Methods
Peginterferon alpha 2b (1.5 μg/kg per week) plus weight-based ribavirin (600–1,000 mg/day) were administered to 141 patients with genotype-1 CHC for 48 weeks. The HCV-RNA loads were measured at baseline, 24 h, week 1, and week 2. The rebound index (RI, viral load at week 1 divided by viral load at 24 h) and the second rebound index (RI-2nd, viral load at week 2 divided by viral load at 24 h) were calculated.
Results
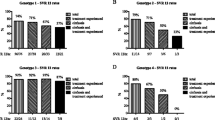
With SVR, the viral load was reduced at 24 h, did not rise during week 1 (RI ≤ 1.0), and was significantly reduced at week 2 (P < 0.05). Viral loads with TR and non-response increased at week 1. The SVR rate was 90% with RI ≤ 1.0, 96% with rapid viral responders, and 93% with RI-2nd <0.7 and week 8 early viral responders. The SVR rate with these 3 groups was 90% and administration for 48 weeks was recommended. With other groups, the SVR rate was 23% and the TR rate was 77%. Administration for 72 weeks was therefore recommended.
Conclusions
We distinguished SVR from TR during treatment using two indices (RI and RI-2nd) and the timing of HCV-RNA negativity.



Similar content being viewed by others
Abbreviations
- SVR:
-
Sustained virologic response
- TR:
-
Transient response
- NR:
-
Non-response
- RI:
-
Rebound index
- RI-2nd:
-
Second rebound index
- RVR:
-
Rapid viral responder
- W8EVR:
-
Week 8 early viral response
- W12EVR:
-
Week 12 early viral response
- LVR:
-
Late viral responder
- NVR:
-
Non-viral responder
References
Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65.
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82.
Muir AJ, Bornstein JD, Killenberg PG, Atlantic Coast Hepatitis Treatment Group. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–71.
Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, et al. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;334:77–81.
Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, et al. Predictors of viral kinetics to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b. J Med Virol. 2007;79:1686–95.
Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, et al. Predictive factors of early and sustained responses to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b: amino acid substitutions in the core region and low-density lipoprotein cholesterol levels. J Hepatol. 2007;46:403–10.
Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1-infected slow responders. Hepatology. 2007;46:1688–94.
Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–97.
Sanchez-Tapias JM, Diago M, Escartin P, Enriquez J, Romero-Gomez M, Barcena R, et al. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131:451–60.
Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–52.
Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Goncales FL Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–33.
Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:945–60.
Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Sheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20.
Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74:2391–9.
Nomura H, Tanimoto H, Kajiwara E, Shimono J, Maruyama T, Yamashita N, et al. Factors contributing to ribavirin-induced anemia. J Gastroenterol Hepatol. 2004;19:1312–7.
Shiffman ML, Ghany MG, Morgan TR, Wright EC, Everson GT, Lindsay KL, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132:103–12.
Silva M, Poo J, Wagner F, Jackson M, Cutler D, Grace M, et al. A randomised trial to compare the pharmacokinetic, pharmacodynamic, and antiviral effects of peginterferon alfa-2b and peginterferon alfa-2a in patients with chronic hepatitis C (COMPARE). J Hepatol. 2006;45:204–13.
Asahina Y, Izumi N, Umeda N, Hosokawa T, Ueda K, Doi F, et al. Pharmacokinetics and enhanced PKR response in patients with chronic hepatitis C treated with pegylated interferon alpha-2b and ribavirin. J Viral Hepat. 2007;14:396–403.
Izumi N, Asahina Y, Kurosaki M, Uchihara M, Nishimura Y, Inoue K, et al. A comparison of the exponential decay slope between PEG-IFN alfa-2b/ribavirin and IFN alfa-2b/ribavirin combination therapy in patients with chronic hepatitis C genotype 1b infection and a high viral load. Intervirology. 2004;47:102–7.
Buti M, Sanchez-Avila F, Lurie Y, Stalgis C, Valdes A, Martell M, et al. Viral kinetics in genotype 1 chronic hepatitis C patients during therapy with 2 different doses of peginterferon alfa-2b plus ribavirin. Hepatology. 2002;35:930–6.
Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci GL, Carretta V, et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology. 2008;47:43–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nomura, H., Miyagi, Y., Tanimoto, H. et al. Effective prediction of outcome of combination therapy with pegylated interferon alpha 2b plus ribavirin in Japanese patients with genotype-1 chronic hepatitis C using early viral kinetics and new indices. J Gastroenterol 44, 338–345 (2009). https://doi.org/10.1007/s00535-009-0008-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0008-z