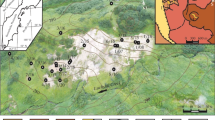
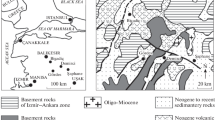
Abstract
The subvolcanic Fohberg phonolite (Kaiserstuhl Volcanic Complex, Germany) is an economic zeolite deposit, formed by hydrothermal alteration of primary magmatic minerals. It is mined due to the high (>40 wt%) zeolite content, which accounts for the remarkable zeolitic physicochemical properties of the ground rock. New mineralogical and geochemical studies are carried out (a) to evaluate the manifestation of hydrothermal alteration, and (b) to constrain the physical and chemical properties of the fluids, which promoted hydrothermal replacement. The alkaline intrusion is characterized by the primary mineralogy: feldspathoid minerals, K-feldspar, aegirine–augite, wollastonite, and andradite. The rare-earth elements-phase götzenite is formed during the late-stage magmatic crystallization. Fluid-induced re-equilibration of feldspathoid minerals and wollastonite caused breakdown to a set of secondary phases. Feldspathoid minerals are totally replaced by various zeolite species, calcite, and barite. Wollastonite breakdown results in the formation of various zeolites, calcite, pectolite, sepiolite, and quartz. Zeolites are formed during subsolidus hydrothermal alteration (<150 °C) under alkaline conditions. A sequence of Ca–Na-dominated zeolite species (gonnardite, thomsonite, mesolite) is followed by natrolite. The sequence reflects an increase in \(\log [(a_{{{\text{Na}}^{ + } }} )/(a_{{{\text{H}}^{ + } }} )]\) and decrease in \(\log [(a_{{{\text{Ca}}^{2 + } }} )/(a_{{{\text{H}}^{ + } }}^{2} )]\) of the precipitating fluid. Low radiogenic 87Sr/86Sr values indicate a local origin of the elements necessary for secondary mineral formation from primary igneous phases. In addition, fractures cut the intrusive body, which contain zeolites, followed by calcite and a variety of other silicates, carbonates, and sulfates as younger generations. Stable isotope analysis of late-fracture calcite indicates very late circulation of meteoric fluids and mobilization of organic matter from surrounding sedimentary units.
















Similar content being viewed by others
References
Akizuki M, Harada K (1988) Symmetry, twinning, and parallel growth of scolecite, mesolite, and natrolite. Am Mineral 73:613–618
Albrecht A (1981) Mineralogische Untersuchungen des Phonoliths vom Fohberg, Kaiserstuhl, mit besonderer Berücksichtigung der mafischen und akzessorischen Minerale. Albert-Ludwigs-University, Freiburg, p 146
Allah BK, Fontan F, Kadar M, Monchoux P, Sørensen H (1998) Reactions between agpaitic nepheline syenitic melts and sedimentary carbonate rocks, exemplified by the Tamazeght complex, Morocco. Geochem Int 36:841–847
Andersen T, Erambert M, Larsen AO, Selbekk RS (2010) Petrology of nepheline syenite pegmatites in the Oslo Rift, Norway: zirconium silicate mineral assemblages as indicators of alkalinity and volatile fugacity in mildly agpaitic magma. J Petrol 51:2303–2325
Baranyi I, Lippold HJ, Todt W (1976) Kalium-Argon-Alterbestimmungen an tertiären Vulkaniten des Oberrheingraben-Gebietes: II Die Alterstraverse vom Hegau nach Lothringen. Oberrhein Geol Abh 25:41–62
Bellezza M, Merlino S, Perchiazzi N (2004) Chemical and structural study of the Zr, Ti-disilicates in the venanzite from Pian di Celle, Umbria, Italy. Eur J Mineral 16:957–969
Birsoy R (2002) Formation of sepiolite–palygorskite and related minerals from solution. Clays Clay Miner 50:736–745
Boles JR, Coombs DS (1977) Zeolite facies alteration of sandstones in the Southland Syncline, New Zealand. Am J Sci 277:982–1012
Boles JR, Franks SG (1979) Clay diagenesis in Wilcox sandstones of Southwest Texas; implications of smectite diagenesis on sandstone cementation. J Sediment Petrol 49:55–70
Boles JR, Surdam RC (1979) Diagenesis of volcanogenic sediments in a Tertiary saline lake; Wagon Bed Formation, Wyoming. Am J Sci 279:832–853
Bourgeois O, Ford M, Diraison M, de Veslud CLC, Gerbault M, Pik R, Ruby N, Bonnet S (2007) Separation of rifting and lithospheric folding signatures in the NW-Alpine foreland. Int J Earth Sci 96:1003–1031
Bucher K, Grapes R (2011) Petrogenesis of metamorphic rocks. Springer, Berlin
Bucher K, Weisenberger T (2013) Fluid-induced mineral composition adjustments during exhumation: the case of Alpine stilbite. Contrib Mineral Petrol 166:1489–1503
Burke WH, Denison RE, Hetherington EA, Koepnick RB, Nelson HF, Otto JB (1982) Variation of seawater 87Sr/86Sr throughout Phanerozoic time. Geology 10:516–519
Callen RA (1984) Clays of the palygorskite–sepiolite group: depositional environment, age and distribution. In: Singer A, Galan E (eds) Developments in sedimentology. Elsevier, Amsterdam, pp 1–37
Christiansen CC, Johnsen O, Makovicky E (2003) Crystal chemistry of the rosenbuschite group. Can Mineral 41:1203–1224
Colella C, Md Gennaro, Aiello R (2001) Use of zeolitic tuff in the building industry. Rev Mineral Geochem 45:551–587
Coulson IM (2003) Evolution of the North Qôroq centre nepheline syenites, South Greenland: alkali-mafic silicates and the role of metasomatism. Mineral Mag 67:873–892
Czygan W (1973) Götzenit, ein komplexes Ti-Zr-Silikat aus dem Kaiserstuhl. Ber Naturforsch Ges Freibg Br 63:5–12
Deer WA, Howie RA, Wise WS, Zussman J (2004) Framework silicates: silica minerals, feldspathoids and the zeolites. The Geological Society of London, London
Dutrow BL, Travis BJ, Gable CW, Henry DJ (2001) Coupled heat and silica transport associated with dike intrusion into sedimentary rock: effects on isotherm location and permeability evolution. Geochim Cosmochim Acta 65:3749–3767
Ferguson LJ, Edgar AD (1978) The petrogenesis and origin of the analcime in the volcanic rocks of the Crowsnest Formation, Alberta. Can J Earth Sci 15:69–77
Fridriksson T, Neuhoff PS, Arnórsson S, Bird DK (2001) Geological constraints on the thermodynamic properties of the stilbite–stellerite solid solution in low-grade metabasalts. Geochim Cosmochim Acta 65:3993–4008
Friedman I, O’Neil JR (1977) Compilation of stable isotope fractionation factors of geochemical interest In: Fleischer M (ed) Data of geochemistry. U.S. Geological Survey Professional Paper
Fuentes F, Aguirre L, Vergara M, Valdebenito L, Fonseca E (2004) Miocene fossil hydrothermal system associated with a volcanic complex in the Andes of central Chile. J Volcan Geotherm Res 138:139–161
Fuhrman ML, Lindsley DH (1988) Ternary-feldspar modeling and thermometry. Am Mineral 73:201–215
Gilg HA, Morteani G, Kostitsyn Y, Preinfalk C, Gatter I, Strieder A (2003) Genesis of amethyst geodes in basaltic rocks of the Serra Geral Formation (Ametista do Sul, Rio Grande do Sul, Brazil): a fluid inclusion, REE, oxygen, carbon, and Sr isotope study on basalt, quartz, and calcite. Miner Depos 38:1009–1025
Grew ES, Locock AJ, Mills SJ, Galuskina IO, Galuskin EV, Hålenius U (2013) Nomenclature of the garnet supergroup. Am Mineral 98:785–811
Güven N, Carney LL (1979) The hydrothermal transformation of sepiolite to stevensite and the effect of added chlorides and hydroxides. Clays Clay Miner 27:253–260
Hauri F (2006) Natural zeolites from southern Germany: applications in concrete In: Bowmann RS, Delap SE (eds) Zeolite’06—7th international conference on the occurrence, properties, and utilization of natrural zeolites. Socorro, New Mexico, USA, p 130
Helgeson HC, Delany JM, Nesbitt HW, Bird DK (1978) Summary and critique of the thermodynamic properties of rock-forming minerals. Am J Sci 278-A:1–229
Hoefs J (1973) Stable isotope geochemistry. Springer, Berlin
Hubberten HW, Katz-Lehnert K, Keller J (1988) Carbon and oxygen isotope investigations in carbonatites and related rocks from the Kaiserstuhl, Germany. Chem Geol 70:257–274
Hüttner R (1996) Tektonik im Grundgebirge. In: Groschopf R, Kessler G, Leiber J, Maus H, Ohmert W, Schreiner A, Wimmenauer W (eds) Geologsiche Karte von Baden-Württemberg 1:50000, Freiburg iBr und Umgebung. Landesamt für Geologie, Rohstoffe und Bergbau Baden-Württemberg, Freiburg, pp 119–228
Ibrahim K (2004) Mineralogy and chemistry of natrolite from Jordan. Clay Miner 39:47–55
Ibrahim K, Hall A (1996) The authigenic zeolites of the Aritayn Volcaniclastic Formation, north-east Jordan. Miner Depos 31:514–522
Isphording WC (1973) Discussion of the occurrence and origin of sedimentary palygorskite–sepiolite deposits. Clays Clay Miner 21:591–601
Johnson JW, Oelkers EH, Helgeson HC (1992) SUPCRT92: a software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Comput Geosci 18:899–947
Kallo D (2001) Applications of natural zeolites in water and wastewater treatment. Rev Mineral Geochem 45:519–550
Karup-Møller S (1969) Xonotlite-, pectolite- and natrolite-bearing fracture veins in volcanic rocks form Nuussuaq, West Greenland. Grønlands Geologiske Undersøgelse 80:4–20
Kassautzki M (1983) Phonolith als puzzolanischer Zumahlstoff in der Zementindustrie. Zem-Kalk-Gips Int 36:688–692
Keller J (1981) Carbonatitic volcanism in the Kaiserstuhl alkaline complex: evidence for highly fluid carbonatitic melts at the earth’s surface. J Volcan Geotherm Res 9:423–431
Keller J (1984) Geochemie und Magmenentwicklung im Kaiserstuhl. Fortschr Mineral 62:116–118
Keller J (2001) Kaiserstuhl alkaline rock-carbonatitic complex—excursion notes. ESF Carbonatite Workshop, Breisach
Kim JS (1985) Petrologie und Geochemie der tephritischen Gesteine im Kaiserstuhl. Albert-Ludwigs-University, Freiburg, p 184
Klaproth MH (1803) Chemische Untersuchung des Natroliths. Ges Naturforsch Freunde Berl Neue Schriften 4:243–248
Kousehlar M, Weisenberger TB, Tutti F, Mirnejad H (2012) Fluid control on low-temperature mineral formation in volcanic rocks of Kahrizak, Iran. Geofluids 12:295–311
Kraml M, Pik R, Rahn M, Selbekk R, Carignan J, Keller J (2006) A new multi-mineral age reference material for 40Ar/39Ar, (U–Th)/He and fission track dating methods: the Limberg t3 tuff. Geostand Geoanal Res 30:73–86
Kristmannsdóttir H, Tómasson J (1978) Zeolite zones in geothermal areas in Iceland. In: Sand LB, Mumpton FA (eds) Natural zeolites: occurrence, properties, usa. Pergamon Press, New York, pp 277–284
Land LS (1987) The major ion chemistry of saline brines in sedimentary basins. AIP Conf Proc 154:160–179
Leggo PJ, Ledesert B (2001) Use of organo-zeolitic fertilizer to sustain plant growth and stabilize metallurgical and mine-waste sites. Mineral Mag 65:563–570
Leggo PJ, Ledesert B, Day J (2010) Organo-zeolitic treatment of mine waste to enhance the growth of vegetation. Eur J Mineral 22:813–822
Lippolt HJ, Todt W, Horn P (1974) Apparent potassium–argon ages of Lower Tertiary Rhine Graben volcanics. In: Illies H, Fuchs K (eds) Approaches to taphrogenesis. Schweizerbart, Stuttgart, pp 213–221
Machiels L, Garcés D, Snellings R, Vilema W, Morante F, Paredes C, Elsen J (2014) Zeolite occurrence and genesis in the Late-Cretaceous Cayo arc of Coastal Ecuador: evidence for zeolite formation in cooling marine pyroclastic flow deposits. Appl Clay Sci 87:108–119
Markl G, Marks M, Schwinn G, Sommer H (2001) Phase equilibrium constraints on intensive crystallization parameters of the Ilímaussaq Complex, South Greenland. J Petrol 42:2231–2257
Marks M, Markl G (2001) Fractionation and assimilation processes in the alkaline augite syenite unit of the Ilímaussaq Intrusion, South Greenland, as deduced from phase equilibria. J Petrol 42:1947–1969
Marzi E (1983) Die Mineralien des Fohbergs bei Bötzingen (Oberschaffhausen) im Kaiserstuhl. Der Aufschluss 34:205–214
Mercurio M, Mercurio V, de Gennaro B, de Gennaro M, Grifa C, Langella A, Morra V (2010) Natural zeolites and white wines from Campania Region: a new approach for solving some oenological problems. Period Mineral 79:95–112
Mercurio M, Langella A, Cappelletti P, de Gennaro B, Monetti V, de Gennaro M (2012) May the use of Italian volcanic zeolite-rich tuffs as additives in animal diet represent a risk for the human health? Period Mineral 81:393–407
Müller W, Shelley M, Miller P, Broude S (2009) Initial performance metrics of a new custom-designed ArF excimer LA-ICPMS system coupled to a two-volume laser-ablation cell. J Anal At Spectrom 24:209–214
Mumpton FA, Roy R (1958) New data on sepiolite and attapulgite. Clays Clay Miner 5:136–143
Neuhoff PS (2000) Thermodynamic properties and parageneses of rock-forming zeolites. Stanford University, Stanford, p 240
Neuhoff PS, Watt WS, Bird DK, Pedersen AK (1997) Timing and structural relations of regional zeolite zones in basalts of the East Greenland continental margin. Geology 25:803–806
Neuhoff PS, Fridriksson T, Arnorsson S, Bird DK (1999) Porosity evolution and mineral paragenesis during low-grade metamorphism of basaltic lavas at Teigarhorn, eastern Iceland. Am J Sci 299:467–501
Neuhoff PS, Fridriksson T, Bird DK (2000) Zeolite parageneses in the north atlantic igneous province: implications for geotectonics and groundwater quality of basaltic crust. Int Geol Rev 42:15–44
Neuhoff PS, Hovis GL, Balassone G, Stebbins JF (2004) Thermodynamic properties of analcime solid solutions. Am J Sci 304:21–66
Neuhoff PS, Rogers KL, Stannius LS, Bird DK, Pedersen AK (2006) Regional very low-grade metamorphism of basaltic lavas, Disko–Nuussuaq region, West Greenland. Lithos 92:33–54
Pabalan RT, Bertetti FP (1999) Experimental and modeling study of ion exchange between aqueous solutions and the zeolite mineral clinoptilolite. J Solut Chem 28:367–393
Pabalan RT, Bertetti FP (2001) Cation-exchange properties of natural zeolites. Rev Mineral Geochem 45:453–518
Passaglia E (1970) The crystal chemistry of chabazite. Am Mineral 55:1278–1301
Passaglia E, Sheppard RA (2001) The Crystal chemistry of zeolites. Rev Mineral Geochem 45:69–116
Pauwels H, Fouillac C, Fouillac A-M (1993) Chemistry and isotopes of deep geothermal saline fluids in the Upper Rhine Graben: origin of compounds and water–rock interactions. Geochim Cosmochim Acta 57:2737–2749
Person M, Garven G (1992) Hydrologic constraints on petroleum generation within continental rift basins; theory and application to the Rhine Graben. AAPG Bull 76:468–488
Rankenburg K, Lassiter JC, Brey G (2004) Origin of megacrysts in volcanic rocks of the Cameroon volcanic chain—constraints on magma genesis and crustal contamination. Contrib Mineral Petrol 147:129–144
Rogers KL, Neuhoff PS, Pedersen AK, Bird DK (2006) CO2 metasomatism in a basalt-hosted petroleum reservoir, Nuussuaq, West Greenland. Lithos 92:55–82
Ross M, Flohr MJK, Ross DR (1992) Crystalline solution series and order–disorder within the natrolite mineral group. Am Mineral 77:685–703
Sahama TG, Hytönen K (1957) Götzenite and combeite, two new silicates from the Belgian Congo. Mineral Mag 31:503–510
Schilling J, Marks MAW, Wenzel T, Vennemann T, Horváth L, Tarassoff P, Jacob DE, Markl G (2011) The magmatic to hydrothermal evolution of the intrusive Mont Saint-Hilaire Complex: insights into the late-stage evolution of peralkaline rocks. J Petrol 52:2147–2185
Schleicher H, Keller J, Kramm U (1990) Isotope studies on alkaline volcanics and carbonatites from the Kaiserstuhl, Federal Republic of Germany. Lithos 26:21–35
Schleicher H, Baumann A, Keller J (1991) Pb isotopic systematics of alkaline volcanic rocks and carbonatites from the Kaiserstuhl, Upper Rhine rift valley, F.R.G. Chem Geol 93:231–243
Schreiner A (1996) Tektonik der Vorbergzone und der Oberrheinebene. In: Groschopf R, Kessler G, Leiber J, Maus H, Ohmert W, Schreiner A, Wimmenauer W (eds) Geologsiche Karte von Baden-Württemberg 1:50000, Freiburg iBr und Umgebung. Landesamt für Geologie, Rohstoffe und Bergbau Baden-Württemberg, Freiburg, pp 229–241
Schultz JL, Boles JR, Tilton GR (1989) Tracking calcium in the San Joaquin basin, California: a strontium isotopic study of carbonate cements at North Coles Levee. Geochim Cosmochim Acta 53:1991–1999
Schwinn G, Wagner T, Baatartsogt B, Markl G (2006) Quantification of mixing processes in ore-forming hydrothermal systems by combination of stable isotope and fluid inclusion analyses. Geochim Cosmochim Acta 70:965–982
Sjöqvist ASL, Cornell DH, Andersen T, Erambert M, Ek M, Leijd M (2013) Three compositional varieties of rare-earth element ore: eudialyte-group minerals from the Norra Kärr Alkaline Complex, Southern Sweden. Minerals 3:94–120
Stober G (1955) Kleintektonische Untersuchungen im Gebiet des Kaiserstuhls und des Limberges. Albert-Ludwigs-University, Freiburg, p 71
Sukheswala RN, Avasia RK, Gangopadhyay M (1974) Zeolites and associated secondary minerals in the Deccan Traps of Western India. Mineral Mag 39:658–671
Surdam RC, Boles JR (1979) Diagenesis of volcanic sandstones. SEPM Spec Publ 26:227–442
Taylor HP Jr, Frechen J, Degens ET (1967) Oxygen and carbon isotope studies of carbonatites from the Laacher See District, West Germany and the Alnö District, Sweden. Geochim Cosmochim Acta 31:407–430
Tchernev DI (2001) Natural zeolites in solar energy heating, cooling, and energy storage. Rev Mineral Geochem 45:589–617
Vetter O (1938) Die Eruptivgesteine das Hegaus, ihre hydrothermalen Umwandlungen und Neubildungen. Neues Jb Miner Geol Abh 73:79–136
Vuorinen JH, Hålenius U, Whitehouse MJ, Mansfeld J, Skelton ADL (2005) Compositional variations (major and trace elements) of clinopyroxene and Ti-andradite from pyroxenite, ijolite and nepheline syenite, Alnö Island, Sweden. Lithos 81:55–77
Walker GPL (1960) The amygdale minerals in the Tertiary lavas of Ireland. III. Regional distribution. Mineral Mag 32:503–527
Wedepohl KH, Gohn E, Hartmann G (1994) Cenozoic alkali basaltic magmas of western Germany and their products of differentiation. Contrib Mineral Petrol 115:253–278
Weisenberger T, Bucher K (2010) Zeolites in fissures of granites and gneisses of the Central Alps. J Metamorph Geol 28:825–847
Weisenberger T, Bucher K (2011) Mass transfer and porosity evolution during low temperature water–rock interaction in gneisses of the simano nappe: Arvigo, Val Calanca, Swiss Alps. Contrib Mineral Petrol 162:61–81
Weisenberger T, Selbekk R (2009) Multi-stage zeolite facies mineralization in the Hvalfjördur area, Iceland. Int J Earth Sci 98:985–999
Weisenberger T, Spürgin S (2009) Zeolites in alkaline rocks of the Kaiserstuhl Volcanic Complex, SW Germany—new microprobe investigation and the relationship of zeolite mineralogy to the host rock. Geol Belg 12:75–91
Weitkamp J (2000) Zeolites and catalysis. Solid State Ion 131:175–188
Whitney DL, Evans BW (2010) Abbreviations for names of rock-forming minerals. Am Mineral 95(1):185–187
Wimmenauer W (1957) Beiträge zur Petrographie des Kaiserstuhls. Einführung und Teil I: Die Ergußgesteine und Tuffe. Neues Jb Miner Abh 91:131–150
Wimmenauer W (1959a) Beiträge zur Petrographie des Kaiserstuhls. Schluß von Teil I: Die Ergußgesteine und Tuffe. Teil II: Die essexitisch-theralitischen subvulkanischen Intrusivegesteine. Teil III: Die Ganggesteine der essexitischen Fanilie. Neues Jb Miner Abh 93:133–173
Wimmenauer W (1959b) Die Minerale des Kaiserstuhls. Der Aufschluss 10:181–202
Wimmenauer W (1962) Beiträge zur Petrographie des Kaiserstuhls: Teil IV Die Gesteine der phonolithischen Familie. Teil V: Die subvulkanischen Breccien. Neues Jb Miner Abh 98:367–415
Wimmenauer W (1963) Beiträge zur Petrographie des Kaiserstuhls: Teil VI: Die Karbonatite. Teil VII: Zur Petrogenese des Kaiserstuhls. Neues Jb Miner Abh 99:231–276
Wimmenauer W (2003) Geologische Karte von Baden-Württemberg 1:25000, Kaiserstuhl. Landesamt für Geologie, Rohstoffe und Bergbau Baden-Württemberg, Freiburg
Wimmenauer W (2010) Kalkadern in vulkanischen und Sedimentgesteinen des Kaiserstuhls. Mitt bad Landesver Naturkunde Naturschutz 21:49–67
Yagi K, Kikuchi T, Kakuta H (1968) Thermal decomposition of pectolite and its hydrothermal synthesis. J Fac Sci Hokkaido Univ Ser 4 Geol Mineral 14:123–134
Zaitsev AN, Marks MAW, Wenzel T, Spratt J, Sharygin VV, Strekopytov S, Markl G (2012) Mineralogy, geochemistry and petrology of the phonolitic to nephelinitic Sadiman volcano, Crater Highlands, Tanzania. Lithos 152:66–83
Zen E-A (1961) The zeolite facies; an interpretation. Am J Sci 259:401–409
Acknowledgments
We are grateful to Hans G. Hauri Mineralstoffwerke for providing access to the Fohberg quarry and support of this study. We thank Sari Forss for thin section preparation, Leena Palmu for her advice and help with the electron microprobe, and Shenhong Yang for help with Sr-isotopic analyses. We thank Lieven Machiels for his detailed and constructive comments and Wolf-Christian Dullo for his efforts and the editorial handling of the paper. Special thanks to the Holopainen Foundation for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weisenberger, T.B., Spürgin, S. & Lahaye, Y. Hydrothermal alteration and zeolitization of the Fohberg phonolite, Kaiserstuhl Volcanic Complex, Germany. Int J Earth Sci (Geol Rundsch) 103, 2273–2300 (2014). https://doi.org/10.1007/s00531-014-1046-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00531-014-1046-1