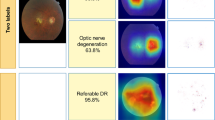
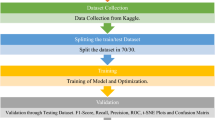
Abstract
Retinal images are a key element for ophthalmologists in diagnosing a variety of eye illnesses. The retina is vulnerable to microvascular changes as a result of many retinal diseases and a variety of research have been done on early diagnosis of medical images to take proper treatment on time. This paper designs an automated deep learning-based non-invasive framework to diagnose multiple eye diseases using colour fundus images. A multi-class eye disease RFMiD dataset was used to develop an efficient diagnostic framework. Multi-class fundus images were extracted from a multi-label dataset and then various augmentation techniques were applied to make the framework robust in real-time. Images were processed according to the network for low computational demand. A multi-layer neural network EyeDeep-Net has been developed to train and test images for diagnosis of various eye problems in which the keystone convolutional neural network extracts relevant features from the input colour fundus image dataset and then processed features were used to make predictive diagnostic decisions. The strength of the EyeDeep-Net is evaluated using multiple statistical parameters and the performance of the proposed model is found to be significantly superior to multiple baseline state-of-the-art models. A comprehensive comparison of the proposed methodology to the most recent methods proves its efficacy in terms of classification and disease identification through digital fundus images.











Similar content being viewed by others
Data availability
The datasets analysed during the current study are publicly available in the Retinal Fundus Multi-Disease Image Dataset (RFMiD repository available at https://riadd.grand-challenge.org/download-all-classes or https://doi.org/10.3390/data6020014.
References
https://www.who.int/publications/i/item/world-report-on-vision, Accessed 25 Feb 2022
Jonas JB, Bourne RRA, White RA, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, Wong TY, Resnikoff S, Taylor HR (2014) visual Impairment and blindness due to macular diseases globally: a systematic review and meta-analysis. Am J Ophthalmol 158:808–815. https://doi.org/10.1016/j.ajo.2014.06.012
Michaud L, Forcier P (2014) Prevalence of asymptomatic ocular conditions in subjects with refractive-based symptoms. J Optom 7:153–160. https://doi.org/10.1016/j.optom.2013.08.003
Méjécase C, Malka S, Guan Z, Slater A, Arno G, Moosajee M (2020) Practical guide to genetic screening for inherited eye diseases. Ther Adv Ophthalmol 12:251584142095459. https://doi.org/10.1177/2515841420954592
Leasher JL, Bourne RRA, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR (2016) Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care 39:1643–1649. https://doi.org/10.2337/dc15-2171
Walton OB, Garoon RB, Weng CY, Gross J, Young AK, Camero KA, Jin H, Carvounis PE, Coffee RE, Chu YI (2016) Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol 134:204. https://doi.org/10.1001/jamaophthalmol.2015.5083
Riazi Esfahani H, Hajizadeh F (2021) Age-related macular degeneration (ARMD). Diagnostics in Ocular Imaging. Springer, Cham, pp 463–476. https://doi.org/10.1007/978-3-030-54863-6_17
Malick H, Din N, Rajput R, Mushtaq B (2021) Cataract surgery in patients with neovascular age related macular degeneration—examination of current practice among UK ophthalmic surgeons. Eye 35:685–686. https://doi.org/10.1038/s41433-020-0863-7
Hayreh SS (1974) Pathogenesis of cupping of the optic disc. Br J Ophthalmol 58:863–876. https://doi.org/10.1136/bjo.58.10.863
Bressler NM (2004) Age-related macular degeneration is the leading cause of blindness. JAMA 291:1900. https://doi.org/10.1001/jama.291.15.1900
Ye H, Zhang Q, Liu X, Cai X, Yu W, Yu S, Wang T, Lu W, Li X, Jin H, Hu Y, Kang X, Zhao P (2014) Prevalence of age-related macular degeneration in an elderly urban Chinese population in china: the Jiangning eye study. Investig Opthalmology Vis Sci 55:6374. https://doi.org/10.1167/iovs.14-14899
Edupuganti VG, Chawla A, Kale A (2018) Automatic optic disk and cup segmentation of fundus images using deep learning. In 2018 25th IEEE international conference on image processing (ICIP), pp 2227–2231
Dias JM, Oliveira CM, da Silva Cruz LA (2014) Retinal image quality assessment using generic image quality indicators. Inf Fusion 19:73–90. https://doi.org/10.1016/j.inffus.2012.08.001
Cui H, Shen S, Gao W, Liu H, Wang Z (2019) Efficient and robust large-scale structure-from-motion via track selection and camera prioritization. ISPRS J Photogramm Remote Sens 156:202–214. https://doi.org/10.1016/j.isprsjprs.2019.08.005
Peli E, Peli T (1989) Restoration of retinal images obtained through cataracts. IEEE Trans Med Imaging 8:401–406. https://doi.org/10.1109/42.41493
Singh A, Dutta MK, ParthaSarathi M, Uher V, Burget R (2016) Image processing based automatic diagnosis of glaucoma using wavelet features of segmented optic disc from fundus image. Comput Methods Programs Biomed 124:108–120. https://doi.org/10.1016/j.cmpb.2015.10.010
Singh A, Dutta MK, Parthasarathi M, Burget R, Riha K (2014) An efficient automatic method of Optic disc segmentation using region growing technique in retinal images. In: 2014 International conference on contemporary computing and informatics (IC3I), pp 480–484. IEEE
Issac SMA, Dutta MK (2018) An automated and robust image processing algorithm for glaucoma diagnosis from fundus images using novel blood vessel tracking and bend point detection. Int J Med Inform 110:52–70. https://doi.org/10.1016/j.ijmedinf.2017.11.015
Soni A, Rai A (2021) Automatic cataract detection using sobel and morphological dilation operation. In: Proceedings of research and applications in artificial intelligence, pp 267–276. https://doi.org/10.1007/978-981-16-1543-6_25
Issac A, Partha Sarathi M, Dutta MK (2015) An adaptive threshold based image processing technique for improved glaucoma detection and classification. Comput Methods Programs Biomed 122:229–244. https://doi.org/10.1016/j.cmpb.2015.08.002
Ganguly S, Ganguly S, Srivastava K, Dutta MK, Parthasarathi M, Burget R, Riha K (2014) An adaptive threshold based algorithm for detection of red lesions of diabetic retinopathy in a fundus image. In: 2014 International conference on medical imaging, m-health and emerging communication systems (MedCom) pp 91–94. https://doi.org/10.1109/MedCom.2014.7005982
Wang W (2022) Further results on mean-square exponential input-to-state stability of stochastic delayed cohen-grossberg neural networks. Neural Process Lett. https://doi.org/10.1007/s11063-022-10974-8
Huang C, Liu B, Yang H, Cao J (2022) Positive almost periodicity on SICNNs incorporating mixed delays and D operator. Nonlinear Anal Model Control 27:1–21
Long S, Chen J, Hu A, Liu H, Chen Z, Zheng D (2020) Microaneurysms detection in color fundus images using machine learning based on directional local contrast. Biomed Eng Online 19:21. https://doi.org/10.1186/s12938-020-00766-3
Rekhi RS, Issac A, Dutta MK, Travieso CM (2017) Automated classification of exudates from digital fundus images. In: 2017 International conference and workshop on bioinspired intelligence (IWOBI) pp 1–6. https://doi.org/10.1109/IWOBI.2017.7985527
García-Floriano A, Ferreira-Santiago Á, Camacho-Nieto O, Yáñez-Márquez C (2019) A machine learning approach to medical image classification: detecting age-related macular degeneration in fundus images. Comput Electr Eng 75:218–229. https://doi.org/10.1016/j.compeleceng.2017.11.008
Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak JAWM, van Ginneken B, Sánchez CI (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60–88. https://doi.org/10.1016/j.media.2017.07.005
Zhao W, Yang J, Sun Y, Li C, Wu W, Jin L, Yang Z, Ni B, Gao P, Wang P, Hua Y, Li M (2018) 3D Deep learning from CT scans predicts tumor invasiveness of subcentimeter pulmonary adenocarcinomas. Cancer Res 78:6881–6889. https://doi.org/10.1158/0008-5472.CAN-18-0696
Yang J, Deng H, Huang X, Ni B, Xu Y (2020) Relational learning between multiple pulmonary nodules via deep set attention transformers. In: 2020 IEEE 17th international symposium on biomedical imaging (ISBI), pp 1875–1878. https://doi.org/10.1109/ISBI45749.2020.9098722
Roy AG, Conjeti S, Karri SPK, Sheet D, Katouzian A, Wachinger C, Navab N (2017) ReLayNet: retinal layer and fluid segmentation of macular optical coherence tomography using fully convolutional networks. Biomed Opt Express 8:3627. https://doi.org/10.1364/BOE.8.003627
Hu K, Zhang Z, Niu X, Zhang Y, Cao C, Xiao F, Gao X (2018) Retinal vessel segmentation of color fundus images using multiscale convolutional neural network with an improved cross-entropy loss function. Neurocomputing 309:179–191. https://doi.org/10.1016/j.neucom.2018.05.011
Playout C, Duval R, Cheriet F (2019) A novel weakly supervised multitask architecture for retinal lesions segmentation on fundus images. IEEE Trans Med Imaging 38:2434–2444. https://doi.org/10.1109/TMI.2019.2906319
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, Venugopalan S, Widner K, Madams T, Cuadros J, Kim R, Raman R, Nelson PC, Mega JL, Webster DR (2016) Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316:2402. https://doi.org/10.1001/jama.2016.17216
Li Z, He Y, Keel S, Meng W, Chang RT, He M (2018) Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology 125:1199–1206. https://doi.org/10.1016/j.ophtha.2018.01.023
. Govindaiah A, Hussain MA, Smith RT, Bhuiyan A (2018) Deep convolutional neural network based screening and assessment of age-related macular degeneration from fundus images. In: 2018 IEEE 15th International symposium on biomedical imaging (ISBI 2018) IEEE pp 1525–1528. https://doi.org/10.1109/ISBI.2018.8363863
Song W, Cao Y, Qiao Z, Wang Q, Yang JJ (2019) An improved semi-supervised learning method on cataract fundus image classification. In: 2019 IEEE 43rd annual computer software and applications conference (COMPSAC): pp 362–367. https://doi.org/10.1109/COMPSAC.2019.10233
Karri SPK, Chakraborty D, Chatterjee J (2017) Transfer learning based classification of optical coherence tomography images with diabetic macular edema and dry age-related macular degeneration. Biomed Opt Express 8:579. https://doi.org/10.1364/BOE.8.000579
Li X, Pang T, Xiong B, Liu W, Liang P, Wang T (2017) Convolutional neural networks based transfer learning for diabetic retinopathy fundus image classification. In: 2017 10th international congress on image and signal processing, biomedical engineering and informatics (CISP-BMEI), IEEE, pp 1–11. https://doi.org/10.1109/CISP-BMEI.2017.8301998
Joshi RC, Dutta MK, Sikora P, Kiac M (2020) Efficient convolutional neural network based optic disc analysis using digital fundus images. In: 2020 43rd international conference on telecommunications and signal processing (TSP), IEEE pp 533–536. https://doi.org/10.1109/TSP49548.2020.9163560.
Sarki R, Ahmed K, Wang H, Zhang Y (2020) Automated detection of mild and multi-class diabetic eye diseases using deep learning. Heal Inf Sci Syst 8:32. https://doi.org/10.1007/s13755-020-00125-5
Sahlsten J, Jaskari J, Kivinen J, Turunen L, Jaanio E, Hietala K, Kaski K (2019) Deep learning fundus image analysis for diabetic retinopathy and macular edema grading. Sci Rep 9:10750. https://doi.org/10.1038/s41598-019-47181-w
T. Wu, L. Liu, T. Zhang, X. Wu, Deep learning-based risk classification and auxiliary diagnosis of macular edema, Intell. Med. 6 (2022) 100053. https://doi.org/10.1016/j.ibmed.2022.100053.
Kumar S, Kumar B (2022) Automatic early glaucoma detection by extracting parapapillary atrophy and optic disc from fundus image using SVM. Multimed Tools Appl. https://doi.org/10.1007/s11042-021-11023-7
Purna Chandra Reddy V, Gurrala KK (2022) Joint DR-DME classification using deep learning-CNN based modified grey-wolf optimizer with variable weights. Biomed Signal Process Control 73:103439
Veena HN, Muruganandham A, Senthil Kumaran T (2022) A novel optic disc and optic cup segmentation technique to diagnose glaucoma using deep learning convolutional neural network over retinal fundus images. J King Saud Univ Comput Inf Sci 34:6187–6198. https://doi.org/10.1016/j.jksuci.2021.02.003
Li F, Wang Y, Xu T, Dong L, Yan L, Jiang M, Zhang X, Jiang H, Wu Z, Zou H (2022) Deep learning-based automated detection for diabetic retinopathy and diabetic macular oedema in retinal fundus photographs. Eye 36:1433–1441. https://doi.org/10.1038/s41433-021-01552-8
Pascal L, Perdomo OJ, Bost X, Huet B, Otálora S, Zuluaga MA (2022) Multi-task deep learning for glaucoma detection from color fundus images. Sci Rep 12:12361. https://doi.org/10.1038/s41598-022-16262-8
Pachade S, Porwal P, Thulkar D, Kokare M, Deshmukh G, Sahasrabuddhe V, Giancardo L, Quellec G, Mériaudeau F (2021) Retinal fundus multi-disease image dataset (RFMiD): a dataset for multi-disease detection research. Data 6:14. https://doi.org/10.3390/data6020014
Ioffe S, Szegedy C (2015) Batch normalization: accelerating deep network training by reducing internal covariate shift. http://arxiv.org/abs/1502.03167
Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R (2014) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15:1929–1958
Agarap AF (2018) Deep learning using rectified linear units (ReLU). http://arxiv.org/abs/1803.08375
Nwankpa C, Ijomah W, Gachagan A, Marshall S (2018) Activation functions: comparison of trends in practice and research for deep learning. http://arxiv.org/abs/1811.03378
Simonyan K, Zisserman A (2014) Very deep convolutional networks for large-scale image recognition. http://arxiv.org/abs/1409.1556
Krizhevsky A, Sutskever I, Hinton GE (2017) ImageNet classification with deep convolutional neural networks. Commun ACM 60:84–90. https://doi.org/10.1145/3065386
Szegedy C, Ioffe S, Vanhoucke V, Alemi A (2016) Inception-v4, inception-ResNet and the impact of residual connections on learning. http://arxiv.org/abs/1602.07261
Yu X, Kang C, Guttery DS, Kadry S, Chen Y, Zhang Y-D (2020) ResNet-SCDA-50 for breast abnormality classification. IEEE/ACM Trans Comput Biol Bioinforma. 18:94–102. https://doi.org/10.1109/tcbb.2020.2986544
Dosovitskiy A, Beyer L, Kolesnikov A, Weissenborn D, Zhai X, Unterthiner T, Dehghani M, Minderer M, Heigold G, Gelly S, Uszkoreit J (2020) An image is worth 16x16 words: transformers for image recognition at scale. http://arxiv.org/abs/2010.11929
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sengar, N., Joshi, R.C., Dutta, M.K. et al. EyeDeep-Net: a multi-class diagnosis of retinal diseases using deep neural network. Neural Comput & Applic 35, 10551–10571 (2023). https://doi.org/10.1007/s00521-023-08249-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-023-08249-x