Abstract
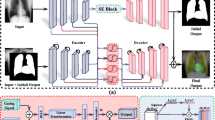
Chest X-ray images (CXRs) are the most common radiological examination tool for screening and diagnosis of cardiac and pulmonary diseases. The automatic segmentation of anatomical structures in CXRs is critical for many clinical applications. However, existing deep models work on severely down-sampled images (commonly \(256\times 256\) pixels), reducing the quality of the contours of the resulting segmentation and negatively affecting the possibilities of such methods to be effectively used in a real environment. In this paper, we study multi-organ (clavicles, lungs, and hearts) segmentation, one of the most important problems in semantic understanding of CXRs. We completely avoid down-sampling in images up to \(1024\times 1024\) (as in the JSRT dataset), and we diminish its impact in higher resolutions via network architecture simplification without a significant loss in the accuracy. To do so, we propose four different convolutional models by introducing structural changes to the baselines employed (U-Net and InvertedNet) as well as by integrating several techniques barely used by CXRs segmentation algorithms, such as instance normalization and atrous convolution. We also compare single-class and multi-class strategies to elucidate which approach is the most convenient for this problem. Our best proposal, X-Net+, outperforms nine state-of-the-art methods on clavicles and lungs obtaining a Dice similarity coefficient of 0.938 and 0.978, respectively, employing a tenfold cross-validation protocol. The same architecture yields comparable results to the state of the art in heart segmentation with a Dice value of 0.938. Finally, its reduced version, RX-Net+, obtains similar results but with a significant reduction in memory usage and training time.



Similar content being viewed by others
Notes
Search performed the September 8, 2018, using the keywords [TITLE-ABS-KEY (chest AND X-ray AND segmentation) OR TITLE-ABS-KEY (chest AND radiograph AND segmentation) AND NOT TITLE-ABS-KEY (computed AND tomography)].
According to the International Agency for Research on Cancer, lung cancer was the most common cause of cancer death in 2015 with 1.69 million deaths.
References
Staffan S, Ostensen H, Pettersson H (2003) The WHO manual of diagnostic imaging: radiographic technique and projections, vol 2. World Health Organization, Geneva
Daffner RH, Hartman M (2013) Clinical radiology: the essentials. Lippincott Williams & Wilkins, Philadelphia
Rigby D-M, Hacking L (2018) Interpreting the chest radiograph. Anaesth Intensive Care 19(2):50–54
Van Ginneken B, Ter Haar Romeny BM, Viergever MA (2001) Computer-aided diagnosis in chest radiography: a survey. IEEE Trans Med Imaging 20(12):1228–1241
NHS England (2016) Diagnostic imaging dataset annual statistical release 2015/2016
Laserson J, Lantsman CD, Cohen-Sfady M, Tamir I, Goz E, Brestel C, Bar S, Atar M, Elnekave E (2018) TextRay: mining clinical reports to gain a broad understanding of chest X-rays. In: MICCAI, pp 553–561
Schilham AMR, van Ginneken B, Loog M (2006) A computer-aided diagnosis system for detection of lung nodules in chest radiographs with an evaluation on a public database. Med Image Anal 10(2):247–258
Mittal A, Hooda R, Sofat S (2017) Lung field segmentation in chest radiographs: a historical review, current status, and expectations from deep learning. IET Image Process 11(11):937–952
Tsakok MT, Gleeson FV (2018) The chest radiograph in heart disease. Medicine 46(8):453–457
Kapoor S, Tiwari A, Kapoor S (2008) Primary tumours and tumorous lesions of clavicle. Int Orthop 32(6):829
Gómez O, Ibáñez O, Valsecchi A, Cordón O, Kahana T (2018) 3D–2D silhouette-based image registration for comparative radiography-based forensic identification. Pattern Recognit 83:469–480
Bruno MA, Walker EA, Abujudeh HH (2015) Understanding and confronting our mistakes: the epidemiology of error in radiology and strategies for error reduction. Radiographics 35(6):1668–1676
Robinson PJ, Wilson D, Coral A, Murphy A, Verow P (1999) Variation between experienced observers in the interpretation of accident and emergency radiographs. Br J Radiol 72(856):323–330
Brady A, Laoide RÓ, McCarthy P, McDermott R (2012) Discrepancy and error in radiology: concepts, causes and consequences. Ulst Med J 81(1):3
Toriwaki J-I, Suenaga Y, Negoro T, Fukumura T (1973) Pattern recognition of chest X-ray images. Comput Vis Graph 2(3):252–271
Wechsler H, Sklansky J (1977) Finding the rib cage in chest radiographs. Pattern Recognit 9(1):21–30
Zhu Y, Prummer S, Wang P, Chen T, Comaniciu D, Ostermeier M (2009) Dynamic layer separation for coronary DSA and enhancement in fluoroscopic sequences. In: MICCAI, pp 877–884
Ronneberger O, Fischer P, Brox T(2015) U-Net: convolutional networks for biomedical image segmentation. In: MICCAI, pp 234–241
Ter Bram Van Ginneken BM, Romeny H, Viergever MA (2001) Computer-aided diagnosis in chest radiography: a survey. IEEE Trans Med Imaging 20(12):1228–1241
Shao Y, Gao Y, Guo Y, Shi Y, Yang X, Shen D (2014) Hierarchical lung field segmentation with joint shape and appearance sparse learning. IEEE Trans Med Imaging 33(9):1761–1780
Yang W, Liu Y, Lin L, Yun Z, Zhentai L, Feng Q, Chen W (2018) Lung field segmentation in chest radiographs from boundary maps by a structured edge detector. IEEE J Biomed Health Inform 22(3):842–851
Boussaid H, Kokkinos I, Paragios N (2014) Discriminative learning of deformable contour models. In: ISBI, IEEE, pp 624–628
Hogeweg L, Sánchez CI, de Jong PA, Maduskar P, van Ginneken B (2012) Clavicle segmentation in chest radiographs. Med Image Anal 16(8):1490–1502
Novikov AA, Lenis D, Major D, Hladuvka J, Wimmer M, Bühler K (2018) Fully convolutional architectures for multiclass segmentation in chest radiographs. IEEE Trans Med Imaging 37(8):1865–1876
Shiraishi J, Katsuragawa S, Ikezoe J, Matsumoto T, Kobayashi T, Komatsu K, Matsui M, Fujita H, Kodera Y, Doi K (2000) Development of a digital image database for chest radiographs with and without a lung nodule: receiver operating characteristic analysis of radiologists’ detection of pulmonary nodules. AJR Am J Roentgenol 174(1):71–74
Van Ginneken B, Stegmann MB, Loog M (2006) Segmentation of anatomical structures in chest radiographs using supervised methods: a comparative study on a public database. Med Image Anal 10(1):19–40
Ulyanov D, Vedaldi A, Lempitsky VS (2016) Instance normalization: the missing ingredient for fast stylization. arxiv:1607.08022
Chen L-C, Papandreou G, Kokkinos I, Murphy K, Yuille AL (2016) DeepLab: semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected CRFs. arxiv:1606.00915,
Withey DJ, Koles ZJ (2007) Medical image segmentation: methods and software. In: NFSI-ICFBI, pp 140–143
Waseem Khan M (2014) A survey: image segmentation techniques. Int J Future Comput Commun 3(2):89
Smistad E, Falch TL, Bozorgi M, Elster AC, Lindseth F (2015) Medical image segmentation on GPUs: a comprehensive review. Med Image Anal 20(1):1–18
Mesejo P, Ibáñez O, Cordón O, Cagnoni S (2016) A survey on image segmentation using metaheuristic-based deformable models: state of the art and critical analysis. Appl Soft Comput 44:1–29
Cabezas M, Oliver A, Lladó X, Freixenet J, Cuadra MB (2011) A review of atlas-based segmentation for magnetic resonance brain images. Comput Methods Programs Biomed 104(3):e158–e177
Peng B, Zhang L, Zhang D (2013) A survey of graph theoretical approaches to image segmentation. Pattern Recognit 46(3):1020–1038
Garcia-Garcia A, Orts-Escolano S, Oprea S, Villena-Martinez V, Garcia-Rodriguez J (2017) A review on deep learning techniques applied to semantic segmentation. arXiv:1704.06857
Chondro P, Yao C-Y, Ruan S-J, Chien L-C (2018) Low order adaptive region growing for lung segmentation on plain chest radiographs. Neurocomputing 275:1002–1011
Siang Tan K, Mat Isa NA (2011) Color image segmentation using histogram thresholding: fuzzy C-means hybrid approach. Pattern Recognit 44(1):1–15
Bi L, Feng D, Kim J (2018) Dual-path adversarial learning for fully convolutional network (FCN)-based medical image segmentation. Vis Comput 34(6):1043–1052
Goh SK, Abbass HA, Tan KC, Al-Mamun A, Thakor N, Bezerianos A, Li J (2018) Spatio-spectral representation learning for electroencephalographic gait-pattern classification. IEEE Trans Neural Syst Rehabilit Eng 26(9):1858–1867
Pang S, José J, del Coz Z, Yu OL, Díez J (2018) Deep learning and preference learning for object tracking: a combined approach. Neural Process Lett 47(3):859–876
Krizhevsky A, Sutskever I, Hinton GE (2012) Imagenet classification with deep convolutional neural networks. In: Advances in neural information processing systems, pp 1097–1105
Li J, Struzik Z, Zhang L, Cichocki A (2015) Feature learning from incomplete EEG with denoising autoencoder. Neurocomputing 165:23–31
Chen L-C, Papandreou G, Schroff F, Adam H (2017) Rethinking atrous convolution for semantic image segmentation. arXiv:1706.05587
Wang C (2017) Segmentation of multiple structures in chest radiographs using multi-task fully convolutional networks. In: Sharma P, Bianchi FM (eds) SCIA, pp 282–289
Mittal A, Hooda R, Sofat S (2018) LF-SegNet: a fully convolutional encoder–decoder network for segmenting lung fields from chest radiographs. Wirel Pers Commun 101(1):511–529
Ioffe S, Szegedy C (2015) Batch normalization: accelerating deep network training by reducing internal covariate shift. ICML 1:448–456
Jaeger S, Candemir S, Antani S, Wáng Y-XJ, Pu-Xuan L, Thoma G (2014) Two public chest X-ray datasets for computer-aided screening of pulmonary diseases. Quant Imaging Med Surg 4(6):475
Hooda R, Mittal A, Sofat S (2018) An efficient variant of fully-convolutional network for segmenting lung fields from chest radiographs. Wirel Pers Commun 101(3):1559–1579
Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R (2014) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15(1):1929–1958
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. K Dan Vidensk Selsk 5:1–34
Denil M, Shakibi B, Dinh L, De Freitas N et al (2013) Predicting parameters in deep learning. In: Advances in neural information processing systems, pp 2148–2156
Han S, Liu X, Mao H, Pu J, Pedram A, Horowitz MA, Dally WJ (2016) EIE: efficient inference engine on compressed deep neural network. In: ISCA, pp 243–254
Kim Y-D, Park E, Yoo S, Choi T, Yang L, Shin D (2015) Compression of deep convolutional neural networks for fast and low power mobile applications. arXiv:1511.06530
Baiying L, Shan H, Ran L, Cheng B, Hang L, Yi-Hong C, Jie-Zhi C (2018) Segmentation of breast anatomy for automated whole breast ultrasound images with boundary regularized convolutional encoder–decoder network. Neurocomputing 321:178–186
Liu J, Cai J, Chellamuthu K, Bagheri M, Lu L, Summers RM (2018) Cascaded coarse-to-fine convolutional neural networks for pericardial effusion localization and segmentation on ct scans. In: 2018 IEEE 15th international symposium on biomedical imaging (ISBI 2018), pp 1092–1095
Gong K, Liang X, Li Y, Chen Y, Yang M, Lin L (2018) Instance-level human parsing via part grouping network. arXiv:1808.00157
Grana C, Borghesani D, Cucchiara R (2010) Optimized block-based connected components labeling with decision trees. IEEE Trans Image Process 19(6):1596–1609
Simonyan K, Vedaldi A, Zisserman A (2013) Deep inside convolutional networks: visualising image classification models and saliency maps. arXiv:1312.6034
Litjens G, Sánchez CI, Timofeeva N, Hermsen M, Nagtegaal I, Kovacs I, Kaa CH-VD, Bult P, Van Ginneken B, Van Der Laak J (2016) Deep learning as a tool for increased accuracy and efficiency of histopathological diagnosis. Sci Rep 6:26286
Qi CR, Hao S, Mo K, Guibas LJ (2017) Pointnet: deep learning on point sets for 3d classification and segmentation. Proc Comput Vis Pattern Recognit IEEE 1(2):4
Beauchemin M, Thomson KPB, Edwards G (1998) On the Hausdorff distance used for the evaluation of segmentation results. Can J Remote Sens 24(1):3–8
Jaccard P (1912) The distribution of the flora in the alpine zone. 1. New Phytol 11(2):37–50
Lathuilière S, Mesejo P, Alameda-Pineda X, Radu H (2018) A comprehensive analysis of deep regression. arXiv:1803.08450
Gehan EA (1965) A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 52(1–2):203–223
Zhang Z, Luo P, Loy CC, Tang X (2014) Facial landmark detection by deep multi-task learning. In: ECCV, pp 94–108
Wu Z, Valentini-Botinhao C, Watts O, King S (2015) Deep neural networks employing multi-task learning and stacked bottleneck features for speech synthesis. In: ICASSP, pp 4460–4464
Myles H, Douglas AW (1999) Nonparametric statistical methods. Wiley, New York
Xiong J, Shao Y, Ma J, Ren Y, Wang Q, Zhao J (2017) Lung field segmentation using weighted sparse shape composition with robust initialization. Med Phys 44(11):5916–5929
Milletari F, Navab N, Ahmadi S-A (2016) V-net: fully convolutional neural networks for volumetric medical image segmentation. In: 3DV, IEEE, pp 565–571
Luo J, Zhang H, Zhou H, Xie C, Wu J, Lin W (2019) ThiNet: pruning CNN filters for a thinner net. IEEE Trans Pattern Anal Mach Intell 41(10):2525–2538
Acknowledgements
This research was supported by the Spanish Ministerio de Economía y Competividad under the NEWSOCO project [Grant Number TIN2015-67661-P], including European Development Regional Funds (EDRF). This work was also supported by the Spanish Ministry of Science, Innovation and Universities, and European Regional Development Funds (ERDF) under grant EXASOCO (PGC2018-101216-B-I00). Mr. Gómez’s work was supported by Spanish MECD FPU Grant [Grant Number FPU14/02380]. Pablo Mesejo is funded by the European Commission H2020-MSCA-IF-2016 through the Skeleton-ID Marie Curie Individual Fellowship [Grant Number 746592]. We acknowledge the support of NVIDIA Corporation with the donation of the Titan X Pascal GPU used for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gómez, O., Mesejo, P., Ibáñez, O. et al. Deep architectures for high-resolution multi-organ chest X-ray image segmentation. Neural Comput & Applic 32, 15949–15963 (2020). https://doi.org/10.1007/s00521-019-04532-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-019-04532-y