Abstract
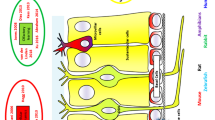
The sense of smell, called olfaction, involves the detection and perception of different odors, and allows identifying food, mates, predators, danger etc. For both humans and animals, it is one of the important means by which they communicate with the environment. This odor-detecting system is called olfactory bulb and is located in the limbic region of the brain. Its functionality is based on neurons, primarily mitral and granule cells, and communication among them. This process is very complex and involves different types of neurotransmitters. The basic function of neurotransmitters is the realization of communication processes between neurons. Additionally, they are responsible for the efficient and accurate processing of the information, as well as for the generation of cellular changes, which corresponds to the memory functionality. In our work, we simulate the main types of chemicals of the olfactory bulb using spiking neuron model: gamma-aminobutyric acid (GABA), N-methyl-d-aspartate (NMDA) and alpha-amino-3-hydroxi-5-methylisoxasole-propionionate (AMPA). In this relatively unexplored area of research (from computing prospective), we design an architecture and experimentally analyze simulation results referring to available biological research and established biophysiological data. We provide the description of different neurotransmitters and their dynamics. The main focus of our work is to analyze the neurotransmitter effects based on the computational simulations corresponding to the biological environment in the olfactory bulb. The results of our work agree with the biological description of the simulated neurotransmitters as well as with experimental results in the biophysiological area.















Similar content being viewed by others
References
Segev I (1999) Taming time in the olfactory bulb. Neuroscience 2:1041–1043
Shepherd GM (1998) The synaptic organization of the brain. Oxford University Press, Oxford
Valova I, Gueorguieva N, Kosugi Y (2004) Oscillation-driven neural network for simulation of olfactory system. Neural Comput Appl 13(1):65–79
Mori K (1987) Membrane and synaptic properties of identified neurons in the olfactory bulb. Prog Neurobiol 29:275–320
Gerstner W, Kistler WM (2002) Spiking neuron models, single neurons, populations, plasticity. Cambridge University Press, Cambridge
http://www.lsm.tugraz.at/csim/index.html
La Camera G, Rauch A, Lüscher H-R, Senn W, Fusi S (2004) Minimal models of adapted neuronal response to in vivo-like input currents. Neural Comput 16(10):2101–2124
Natschläger T, Maass W (2001) Finding the key to a synapse. In: Leen TK, Dietterich TG, Tresp V (eds) Advances in neural information processing systems (NIPS ‘2000), vol 13, pp 138–144, Cambridge
Schoppa NE, Westbrook GL (1999) Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Nat Neurosci 2:1106–1113
Urban NN, Sakmann B (2002) Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol 542:355–367
Friedman D, Strowbridge BW (2000) Functional role of NMDA autoreceptors in olfactory mitral cells. J Neurophysiol 84:39–50
Carlston GC, Shipley MT, Keller A (2000) Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci 20(5):2011–2021
Halabinsky B, Friedman D (2000) Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci 20(13):5124–5134
Davison AP, Feng J, Brown D (2000) A reduced compartmental model of the mitral cell for use in network models of the olfactory bulb. Brain Res Bull 51:393–399
Davison AP, Feng J, Brown D (2003) Dendrodendritic inhibition and simulated odor responses in a detailed olfactory bulb network model. J Neurophysiol 90:1921–1935
Clements JD, Feltz A, Sahara J, Westbrook GL (1998) Activation kinetics of AMPA receptor channels reveal the number of functional agonist binding sites. J Neurosci 18:119–127
Davison A, Feng J, Brown D (2001) Spike synchronization in a biophysically-detailed model of the olfactory bulb. Neurocomputing 38–40:515–521
Schoppa NE, Kinzie JM, Sahara J, Segerson TP, Westbrook GL (1998) Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J Neurosci 18(17):6790–6802
Smith TC, Jahr CI (2002) Self-inhibition of olfactory bulb neurons. Nat Neurosci 5:760–766
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valova, I., Lapteva, O. & Gueorguieva, N. Modeling of neurotransmitter effects in olfactory bulb. Neural Comput & Applic 16, 341–353 (2007). https://doi.org/10.1007/s00521-006-0059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-006-0059-5