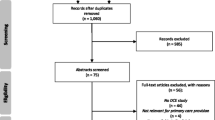
Abstract
Purpose
To ensure the safe use of oral anticancer drugs, oncology pharmacy consultations (OPCs) have been established in France. They are conditioned by the needs, expectations, and involvement of the patients in their care. Thus, it is essential to elicit their preferences. The discrete-choice experiment (DCE) is a method recommended by the ISPOR for such a task. The “selection and validation of attributes and their values” step is fundamental in this process. In this context, the aim of this study was to present our research approach to identify and validate the attributes that characterize an OPC and their values.
Methods
Due to the lack of relevant published data in the literature, the focus-group method was used in accordance with good research practices for the application of conjoint-analysis of the ISPOR. The two-round Delphi method was used to validate the attributes and their values identified by the focus-group method.
Results
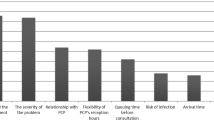
The focus-group method enabled identification of nine attributes. Thirty-seven healthcare professionals at a national level, including 30 pharmacists and seven physicians, were selected to take part in the Delphi procedure. Seven attributes (frequency, planification, operation mode, duration, content, written support, and report) and their values were thus validated.
Conclusion
Based on these results, the next step will be to elicit patient preferences for OPCs and to then shed light on the issues of pharmaceutical support for patients by comparing their preferences with those of informal caregivers and, in particular, those of the healthcare professionals involved in their care.

Similar content being viewed by others
References
The French National Cancer Institute - www.en.ecancer.fr. https://en.e-cancer.fr/. Accessed 25 Apr 2024
Lasala R, Santoleri F (2022) Association between adherence to oral therapies in cancer patients and clinical outcome: a systematic review of the literature. Br J Clin Pharmacol 88(5):1999–2018. https://doi.org/10.1111/bcp.15147
Petit-Jean E, Correard F, Maillan G et al (2019) Pharmaceutical consultations in oncology: French Society for Oncology Pharmacy (Société Francaise de Pharmacie Oncologique – SFPO) guidelines. Eur J Oncol Pharm 2(2):e11. https://doi.org/10.1097/OP9.0000000000000011
Moumjid N, Nguyen F, Bremond A et al (2008) Patients’ preferences and decision-making: state of the art and applications in cancer. Rev Epidemiol Sante Publique 56(Suppl 3):S231-238. https://doi.org/10.1016/j.respe.2008.04.008
Bastiaens H, Van Royen P, Pavlic DR, Raposo V, Baker R (2007) Older people’s preferences for involvement in their own care: a qualitative study in primary health care in 11 European countries. Patient Educ Couns 68(1):33–42. https://doi.org/10.1016/j.pec.2007.03.025
Charles C, Whelan T, Gafni A (1999) What do we mean by partnership in making decisions about treatment? BMJ 319(7212):780–782. https://doi.org/10.1136/bmj.319.7212.780
Bridges JFP, Hauber AB, Marshall D et al (2011) Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health 14(4):403–413. https://doi.org/10.1016/j.jval.2010.11.013
Reed Johnson F, Lancsar E, Marshall D et al (2013) Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health 16(1):3–13. https://doi.org/10.1016/j.jval.2012.08.2223
Hauber AB, González JM, Groothuis-Oudshoorn CGM et al (2016) Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health 19(4):300–315. https://doi.org/10.1016/j.jval.2016.04.004
Berchi C, Launoy G (2007) Principle, strengths and weaknesses of discrete choice modelling for eliciting public preferences for pare. Rev Epidemiol Sante Publique 55(2):133–139. https://doi.org/10.1016/j.respe.2006.11.002
Riboulet M, Clairet AL, Bennani M, et al (2024) Patient preferences for pharmacy services: a systematic review of studies based on discrete-choice experiments. Patient17(1):13–24. https://doi.org/10.1007/s40271-023-00652-9
Kawaguchi T, Azuma K, Yamaguchi T et al (2014) Preferences for pharmacist counselling in patients with breast cancer: a discrete choice experiment. Biol Pharm Bull 37(11):1795–1802. https://doi.org/10.1248/bpb.b14-00452
Wong LP (2008) Focus group discussion: a tool for health and medical research. Singapore Med J 49(3):256–260 (quiz 261)
Linstone HA, Turoff M (1975) The Delphi method: techniques and applications. J Mark Res. https://doi.org/10.2307/3150755
Diamond IR, Grant RC, Feldman BM et al (2014) Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 67(4):401–409. https://doi.org/10.1016/j.jclinepi.2013.12.002
Jones J, Hunter D (1995) Consensus methods for medical and health services research. BMJ 311(7001):376–380. https://doi.org/10.1136/bmj.311.7001.376
Murphy MK, Black NA, Lamping DL et al (1998) Consensus development methods, and their use in clinical guideline development. Health Technol Assess 2(3):1–88
Fitch K, Bernstein S, Aguilar M, et al (2001) The RAND/UCLA appropriateness method user’s manual. RAND corporation
Bridges JFP, de Bekker-Grob EW, Hauber B et al (2023) A roadmap for increasing the usefulness and impact of patient-preference studies in decision making in health: a good practices report of an ISPOR task force. Value Health 26(2):153–162. https://doi.org/10.1016/j.jval.2022.12.004
Acknowledgements
French Implication Expert Panel: Dr. Virginie Andre (Centre Hospitalier Universitaire de Tours), Dr. Jeanne Briet (Centre Hospitalier de Valenciennes), Dr. Michael Bringuier (Institut Curie, Paris), Dr. Régine Chevrier (Centre Jean Perrin, Clermont-Ferrand), Dr. Florian Correard (Hôpital La Timone, Marseille), Dr. Amélie Cransac (Centre Hospitalier Universitaire de Dijon), Dr. Alice Danckaert (Centre Hospitalier d’Arras), Dr. Françoise Decrozals (Institut Sainte Catherine, Avignon), Dr. Elise Deluche (Centre Hospitalier Universitaire de Limoges), Dr. Catherine Devys (Institut de Cancérologie de l’Ouest), Dr. Nelly Etienne-Selloum (Institut de Cancérologie Strasbourg Europe), Dr. Raphaëlle Fanciullino (Hôpital de la Conception, Marseille), Dr. Julie Fulcrand (Centre Hospitalier de Valenciennes), Dr. Vincent Goldschmidt (Institut Gustave Roussy, Paris), Dr. Jérémy Jost (Centre Hospitalier Universitaire de Limoges), Dr. Murielle Laudet (Centre Hospitalier Universitaire de Clermont-Ferrand), Dr. Fanny Leenhardt (Insitut du cancer, Montpellier), Dr. Barbara Lortal (Institut Bergonié, Bordeaux), Dr. Isabelle Madelaine (Hôpital Saint-Louis, Paris), Dr. Pierre Nizet (Centre Hospitalier Universitaire de Nantes), Dr. Selim Omrani (Hôpital Nord Franche Comté), Dr. Emeline Orillard (Centre Hospitalier Universitaire de Besançon), Dr. Germain Perrin (Centre Georges Pompidou, Paris), Dr. Sophie Potin (Centre Hospitalier Universitaire de Rennes), Dr. Florent Puisset (Institut Universitaire du Cancer de Toulouse), Dr. Liliane Remenieras (Centre Hospitalier Universitaire de Limoges), Dr. Fanny Rethouze (Centre Oscar Lambret, Lille), Pr. Catherine Rioufol (Centre Hospitalier Universitaire de Lyon), Dr. Audrey Saint-Ghislain (Centre Hospitalier de Dunkerque), Dr. Antonin Schmitt (Centre de Lutte Contre le Cancer Dijon), Pr. Nicolas Simon (Centre Hospitalier Universitaire de Lille), Dr. Florian Slimano (Centre Hospitalier Universitaire de Reims), Dr. Geoffrey Strobbe (Centre Oscar Lambert, Lille), Dr. Aurélie Terrier-Lenglet (Centre Hospitalier Universitaire d’Amiens), Dr. Audrey Thomas (Hôpital Cochin, Paris), Dr. Julie Vardanega (Centre Hospitalier Universitaire de Besançon), Dr. Erika Viel-Truong (Centre Hospitalier Montceau).
Author information
Authors and Affiliations
Consortia
Contributions
Margaux Damerval: data collection, statistical analysis, and writing; Mohammed Bennani: study design, proofreading, and correction; Catherine Rioufol: proofreading and correction; Selim Omrani: proofreading and correction; Margaux Riboulet: proofreading and correction; Nelly Etienne-Selloum: proofreading and correction; Audrey Saint-Ghislain: proofreading and correction; Fanny Leenhardt: proofreading and correction; Antonin Schmitt: proofreading and correction; Nicolas Simon: proofreading and correction; Anne-Laure Clairet: proofreading and correction; Aurélia Meurisse: statistical analysis, proofreading, and correction; Virginie Nerich: study design and supervision, statistical analysis, proofreading, and correction.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is a professional practice evaluation in order to identify the possible area of improvement. It does not concern medical research on human beings and data that allow their identification.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Damerval, M., Bennani, M., Rioufol, C. et al. Attributes for a discrete-choice experiment on preferences of patients for oncology pharmacy consultations. Support Care Cancer 32, 318 (2024). https://doi.org/10.1007/s00520-024-08517-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08517-z