Abstract
Background
Trifluridine/tipiracil (TAS-102) is an oral anticancer drug with adequate efficacy in unresectable colorectal cancer, but frequently also induces chemotherapy-induced nausea and vomiting (CINV). To investigate the occurrence of CINV and antiemetic therapy in patients with colorectal cancer treated with TAS-102 (JASCC-CINV 2001).
Methods
We conducted a multicenter, prospective, observational study in patients with colorectal cancer who received TAS-102 without dose reduction for the first time. Primary endpoint was the incidence of vomiting during the overall period. Secondary endpoints were the incidence of nausea, significant nausea, anorexia, other adverse events (constipation, diarrhea, insomnia, fatigue, dysgeusia) and patient satisfaction. Patient diaries were used for primary and secondary endpoints. All adverse events were subjectively assessed using PRO-CTCAE ver 1.0. and CTCAE ver 5.0.
Results
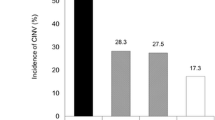
Data from 100 of the 119 enrolled patients were analyzed. The incidence of vomiting, nausea, and significant nausea was 13%, 67%, and 36%, respectively. The incidence of vomiting in patients with and without prophylactic antiemetic therapy were 20.8% and 10.5%, respectively. Prophylactic antiemetics were given to 24% of patients, of whom 70% received D2 antagonists. Multivariate Cox proportional hazards analysis showed that experience of CINV in previous treatment tended to be associated with vomiting (hazard ratio [HR]: 7.13, 95% confidence interval [CI]: 0.87–58.5, P = 0.07), whereas prophylactic antiemetic administration was not (HR: 1.61, 95 CI: 0.50–5.21, P = 0.43). With regard to patient satisfaction, the proportion of patients who were "very satisfied," "satisfied," "slightly satisfied" or "somewhat satisfied" was 81.8%.
Conclusions
The low incidence of vomiting and high patient satisfaction suggest that TAS-102 does not require the use of uniform prophylactic antiemetic treatments. However, patients with the experience of CINV in previous treatment might require prophylactic antiemetic treatment.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Tanaka N, Sakamoto K, Okabe H et al (2014) Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 32:2319–2326
Sakamoto K, Yokogawa T, Ueno H et al (2015) Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2’-deoxy-5-fluorouridine into DNA. Int J Oncol 46:2327–2334
Emura T, Murakami Y, Nakagawa F et al (2004) A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med 13:545–549
Fukushima M, Suzuki N, Emura T et al (2000) Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2’-deoxyribonucleosides. Biochem Pharmacol 59:1227–1236
Mayer RJ, Van Cutsem E, Falcone A et al (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372:1909–1919
Fujii H, Matsuhashi N, Kitahora M et al (2020) Bevacizumab in combination with TAS-102 improves clinical outcomes in patients with refractory metastatic colorectal cancer: a retrospective study. Oncologist 25:e469–e476
Takahashi T, Yamazaki K, Oki E et al (2021) Phase II study of trifluridine/tipiracil plus bevacizumab by RAS mutation status in patients with metastatic colorectal cancer refractory to standard therapies: JFMC51-1702-C7. ESMO Open 6:100093
Pfeiffer P, Yilmaz M, Möller S et al (2020) TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol 21:412–420
Prager GW, Taieb J, Fakih M et al (2023) Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med 388:1657–1667
Kuboki Y, Nishina T, Shinozaki E et al (2017) TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol 18:1172–1181
Hesketh PJ, Kris MG, Basch E et al (2020) Antiemetics: ASCO Guideline Update. J Clin Oncol 38:2782–2797
Aogi K, Takeuchi H, Saeki T et al (2021) Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: Update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol 26:1–17
NCCN clinical practice guidelines in oncology: antiemesis, version 1.2023. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 23 Feb 2023
Jordan K, Chan A, Gralla RJ et al (2023) Emetic risk classification and evaluation of the emetogenicity of antineoplastic agents-updated MASCC/ESMO consensus recommendation. Support Care Cancer 32:53
Dueck AC, Mendoza TR, Mitchell SA et al (2015) Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 1:1051–1059
U.S. Department of Health and Human Services, National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. Available online: https://www.eortc.be/services/doc/ctc/. Accessed 19 Sep 2022
Matsuoka S, Fujii H, Iihara H et al (2023) Emetogenicity and risk factors of nausea and vomiting in patients with metastatic colorectal cancer receiving trifluridine/tipiracil and bevacizumab chemotherapy. Anticancer Res 43:2351–2357
Kanis JA, Johansson H, Oden A et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Rowbottom L, Stinson J, McDonald R et al (2015) Retrospective review of the incidence of monitoring blood glucose levels in patients receiving corticosteroids with systemic anticancer therapy. Ann Palliat Med 4:70–77
Navari RM, Nagy CK, Gray SE (2013) The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21:1655–1663
Yanai T, Iwasa S, Hashimoto H et al (2018) A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 23:382–388
Hashimoto H, Abe M, Tokuyama O et al (2020) Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:242–9
Cao J, Ouyang Q, Wang S et al (2020) Mirtazapine, a dopamine receptor inhibitor, as a secondary prophylactic for delayed nausea and vomiting following highly emetogenic chemotherapy: an open label, randomized, multicenter phase III trial. Investig New Drugs 38:507–514
Maleki A, Ghadiyani M, Salamzadeh J et al (2020) Comparison of mirtazapine and olanzapine on nausea and vomiting following anthracycline-cyclophosphamide chemotherapy regimen in patients with breast cancer. Iran J Pharm Res 19:451–464
Acknowledgements
We thank all the patients and their families for participating in this study. We thank Dr. Takuma Ishihara (Gifu University Hospital) for his support in statistical analysis. We thank the Independent Alliance Data Center, and Ms. Mami Matsumaru (Gifu University Hospital). We would like to express our sincere gratitude to our advisors, Prof. Kazuo Tamura (Fukuoka University School of Medicine), Prof. Toshiaki Saeki (Saitama Medical University International Medical Center), Prof. Keisuke Aiba (The Jikei University School of Medicine), Prof. Mitsue Saito (Juntendo University School of Medicine), Dr. Kenji Okita (Sapporo Medical University School of Medicine) and, Pres. Kazuhiro Yoshida (Gifu University) for their invaluable guidance and support throughout this research project.
Funding
This study did not receive funding from any funding source.
Author information
Authors and Affiliations
Contributions
H.I. and D.W. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: H.I., N.I., J.N., H.H., H.F., and D.W. Acquisition, analysis, or interpretation of data: H.I., N.I., J.N., H.F., M.T., D.W., R.O., D.H., K.T., M.G., T.K., K.S., Y.A., S.T., T.T., K.H., M.C., N.M., M.T., A.H., T.U., H.H., A.K., N.M., and A.S. Drafting of the manuscript: H.F., H.I., and D.W. Critical revision of the manuscript for important intellectual content: H.I., H.F., N.I., J.N., N.H. Statistical analysis: D.W. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
H Fujii has received honoraria for lectures from Ono Pharm, Chugai Pharm, and Taiho Pharm. M Tsuchiya has received honoraria for lectures from Ono Pharm, Chugai Pharm, Taiho Pharm, Sun Pharm and Kyowa Kirin. D Watanabe has received honoraria for lectures from Chugai Pharm. D Hirate has received honoraria for lectures from Taiho Pharm, Daiichi Sankyo, Eli Lilly and Company, Pfizer Eisai, Takeda Pharm, Bristol Myers Squibb, EA Pharma, MSD Japan, Kyowa Kirin, Chugai Pharm, Yakult Honsha, Nippon Kayaku, Eisai, Meiji Seika Pharma, Merck Bio Pharma, AstraZeneca plc and Sun Pharma. Y Ando has received honoraria for lectures from Yakult Honsha, Taiho Pharm, Daiichi Sankyo, Pfizer Eisai. N Matsunami has received honoraria for lectures from Pfizer Eisai, Daiichi Sankyo and Eli Lilly and Company. N Matsuhashi has received both honoraria for lectures and grants made to institution from Taiho Pharm. J Nishimura has received honoraria for lectures from Taiho Pharm. N Inui has received honoraria for lectures from Taiho Pharm, AstraZeneca plc and Chugai Pharm. A Suzuki has received honoraria for lectures from Toa Eiyo, Asahi Kasei Pharma, Daiichi Sankyo, Pfizer Eisai, Nippon Shinyaku, Celltrion Healthcare Japan, Otsuka Pharm, Sandoz, Tsumura, Nipro, Taiho Pharm, Kyowa Kirin, Nippon Chemiphar, Japan Blood Products Organization, Takeda Pharm, and Nippon Boehringer Ingelheim and grants made to institution from Nippon Kayaku, Asahi Kasei Pharma, Chugai Pharm, Taiho Pharm, Daiichi Sankyo, Japan Blood Products Organization, Mochida Pharm, Sun Pharma and consulting fees from Nippon Kayaku. H Iihara has received honoraria for lectures from Taiho Pharm, Chugai Pharma, Yakult Honsha, Astellas Pharma, Eli Lilly and Company, Daiichi Sankyo, AstraZeneca plc, Nippon Kayaku, Ono Pharm, and Nippon Boehringer Ingelheim; and consulting fees from Pfizer Eisai and Taiho Pharm. The other authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujii, H., Tsuchiya, M., Watanabe, D. et al. The emerging emetogenicity of trifluridine/tipiracil (TAS‑102) from patient self-reporting: a multicenter, prospective, observational study. Support Care Cancer 32, 291 (2024). https://doi.org/10.1007/s00520-024-08498-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08498-z