Abstract
Purpose
Treatment decision-making for older adults with acute myeloid leukemia (AML) is complex and preference-sensitive. We sought to understand the patient experience of treatment decision-making to identify specific challenges in shared decision-making to improve clinical care and to inform the development of directed interventions.
Methods
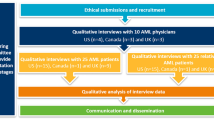
We conducted in-depth interviews with newly diagnosed older (≥ 60 years) adults with AML and their caregivers following a semi-structured interview guide at a public safety net academic hospital. Interviews were digitally recorded, and qualitative thematic analysis was employed to synthesize findings.
Results
Eighteen in-depth interviews were conducted. Age ranged from 62 to 78 years. Patients received intermediate- (50%) or high-intensity (44%) chemotherapy or best supportive care only (6%). Six themes of patient experiences emerged from the analysis: patients (1) felt overwhelmed and in shock at diagnosis, (2) felt powerless to make decisions, (3) felt rushed and unprepared to make a treatment decision, (4) desired to follow oncologist recommendations for treatment, (5) balanced multiple competing factors during treatment decision-making, and (6) desired for ongoing engagement into their care planning. Patients reported many treatment outcomes that were important in treatment decision-making.
Conclusions
Older adults with newly diagnosed AML feel devastated and in shock at their diagnosis which appears to contribute to a feeling of being overwhelmed, unprepared, and rushed into treatment decisions. Because no one factor dominated treatment decision-making for all patients, the use of strategies to elicit individual patient preferences is critical to inform treatment decisions. Interventions are needed to reduce distress and increase a sense of participation in treatment decision-making.

Similar content being viewed by others
References
U. S. National Institutes of Health, National Cancer Institute (2019) Acute myeloid leukemia - cancer stat facts. In: Acute myeloid leukemia statistics. https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 18 Nov 2018
Short NJ, Konopleva M, Kadia TM et al (2020) Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov 10(4):506–525. https://doi.org/10.1158/2159-8290.CD-19-1011
Sorror ML, Storer BE, Fathi AT et al (2021) Multisite 11-year experience of less-intensive vs intensive therapies in acute myeloid leukemia. Blood 138:387–400. https://doi.org/10.1182/blood.2020008812
Jensen CE, Heiling HM, Beke KE et al (2022) Time spent at home among older adults with acute myeloid leukemia receiving azacitidine- or venetoclax-based regimens. Haematol. https://doi.org/10.3324/haematol.2022.280728
Richardson DR, Zhou X, Jensen CE et al (2022) Time at home among older adults with acute myeloid leukemia based on treatment intensity: a SEER-Medicare analysis. JCO 40:6586–6586. https://doi.org/10.1200/JCO.2022.40.16_suppl.6586
Burd A, Levine RL, Ruppert AS et al (2020) Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. https://doi.org/10.1038/s41591-020-1089-8
Richardson DR, Crossnohere NL, Seo J et al (2020) Age at diagnosis and patient preferences for treatment outcomes in AML: a Discrete Choice Experiment to explore meaningful benefits. Cancer Epidemiol Biomarkers Prev 29:942–948. https://doi.org/10.1158/1055-9965.EPI-19-1277
Elwyn G, Durand MA, Song J et al (2017) A three-talk model for shared decision making: multistage consultation process. BMJ 359:j4891. https://doi.org/10.1136/bmj.j4891
Bories P, Lamy S, Simand C et al (2018) Physician uncertainty aversion impacts medical decision making for older patients with acute myeloid leukemia: results of a national survey. Haematologica 103:2040–2048. https://doi.org/10.3324/haematol.2018.192468
Rood JAJ, van Zuuren FJ, Stam F et al (2015) Perceived need for information among patients with a haematological malignancy: associations with information satisfaction and treatment decision-making preferences. Hematol Oncol 33:85–98. https://doi.org/10.1002/hon.2138
Geerts PAF, van der Weijden T, Moser A, Bos GMJ (2020) The perception of shared decision-making in hematology by patients and physicians seems satisfactory, but important steps are still ahead of us. Hemasphere 4:e417. https://doi.org/10.1097/HS9.0000000000000417
Bacher U, Shumilov E, Flach J et al (2018) Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J 8:113. https://doi.org/10.1038/s41408-018-0148-6
LeBlanc TW, Russell NH, Hernandez-Aldama L et al (2022) Patient, family member and physician perspectives and experiences with AML treatment decision-making. Oncol Ther 10(2):421–440. https://doi.org/10.1007/s40487-022-00200-9
Lerner JS, Li Y, Valdesolo P, Kassam KS (2015) Emotion and decision making. Annu Rev Psychol 66:799–823. https://doi.org/10.1146/annurev-psych-010213-115043
Cordova MJ, Riba MB, Spiegel D (2017) Post-traumatic stress disorder and cancer. Lancet Psychiatry 4:330–338. https://doi.org/10.1016/S2215-0366(17)30014-7
Amonoo HL, LeBlanc TW, Kavanaugh AR et al (2021) Posttraumatic stress disorder (PTSD) symptoms in patients with acute myeloid leukemia (AML). Cancer. https://doi.org/10.1002/cncr.33524
LeBlanc TW, Erba HP (2019) Shifting paradigms in the treatment of older adults with AML. Semin Hematol 56:110–117. https://doi.org/10.1053/j.seminhematol.2019.02.002
Loh KP, Abdallah M, Kadambi S et al (2021) Treatment decision-making in acute myeloid leukemia: a qualitative study of older adults and community oncologists. Leuk Lymphoma 62:387–398. https://doi.org/10.1080/10428194.2020.1832662
Puts MTE, Sattar S, McWatters K et al (2017) Chemotherapy treatment decision-making experiences of older adults with cancer, their family members, oncologists and family physicians: a mixed methods study. Support Care Cancer 25:879–886. https://doi.org/10.1007/s00520-016-3476-8
LeBlanc TW, Fish LJ, Bloom CT et al (2017) Patient experiences of acute myeloid leukemia: a qualitative study about diagnosis, illness understanding, and treatment decision-making. Psychooncology 26:2063–2068. https://doi.org/10.1002/pon.4309
Nissim R, Zimmermann C, Minden M et al (2013) Abducted by the illness: a qualitative study of traumatic stress in individuals with acute leukemia. Leuk Res 37:496–502. https://doi.org/10.1016/j.leukres.2012.12.007
Wagner EH, Aiello Bowles EJ, Greene SM et al (2010) The quality of cancer patient experience: perspectives of patients, family members, providers and experts. Qual Saf Health Care 19:484–489. https://doi.org/10.1136/qshc.2010.042374
Deimling GT, Bowman KF, Sterns S et al (2006) Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology 15:306–320. https://doi.org/10.1002/pon.955
Reis BS, Nogueira CM, Meneses AdeFP et al (2023) Experiences of women with advanced cervical cancer before starting the treatment: Systematic review of qualitative studies. Int J Gynaecol Obstet 161:8–16. https://doi.org/10.1002/ijgo.14491
Hasdenteufel M, Quintard B (2022) Dyadic experiences and psychosocial management of couples facing advanced cancer: a systematic review of the literature. Front Psychol 13:827947. https://doi.org/10.3389/fpsyg.2022.827947
Gilligan T, Coyle N, Frankel RM et al (2017) Patient-clinician communication: American Society of Clinical Oncology Consensus Guideline. JCO 35:3618–3632. https://doi.org/10.1200/JCO.2017.75.2311
Klepin HD, Geiger AM, Tooze JA et al (2013) Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 121:4287–4294. https://doi.org/10.1182/blood-2012-12-471680
Min G-J, Cho B-S, Park S-S et al (2022) Geriatric assessment predicts nonfatal toxicities and survival for intensively treated older adults with AML. Blood 139:1646–1658. https://doi.org/10.1182/blood.2021013671
Richardson DR, Oakes AH, Crossnohere NL et al (2021) Prioritizing the worries of AML patients: quantifying patient experience using best-worst scaling. Psychooncology 30:1104–1111. https://doi.org/10.1002/pon.5652
Rood JAJ, Nauta IH, Witte BI et al (2017) Shared decision-making and providing information among newly diagnosed patients with hematological malignancies and their informal caregivers: not “one-size-fits-all.” Psychooncology 26:2040–2047. https://doi.org/10.1002/pon.4414
Watson E, Sanapala C, Cortes A-M et al (2022) Adapting a patient-centered communication tool for older patients with acute myeloid leukemia and their oncologist. Blood Adv 6:5707–5710. https://doi.org/10.1182/bloodadvances.2022008041
LoCastro M, Sanapala C, Wang Y et al (2023) Patient-centered communication tool for older patients with acute myeloid leukemia, their caregivers, and oncologists: a single-arm pilot study. Cancer Med 12:8581–8593. https://doi.org/10.1002/cam4.5547
Cole A, Richardson DR, Adapa K et al (2022) Development of a patient-centered preference tool for patients with hematologic malignancies: protocol for a mixed methods study. JMIR Res Protoc 11:e39586. https://doi.org/10.2196/39586
Cole A, Khasawneh A, Adapa K, Mazur L, Richardson DR (2022) Development of an electronic healthcare tool to elicit patient preferences in older adults diagnosed with hematologic malignancies. In: Gao Q, Zhou J (eds) Human aspects of IT for the aged population. Technology in Everyday Living. HCII 2022. Lecture notes in computer science, vol 13331. Springer, Cham. https://doi.org/10.1007/978-3-031-05654-3_14
El-Jawahri A, LeBlanc TW, Kavanaugh A et al (2020) Multisite randomized trial of integrated palliative and oncology care for patients with acute myeloid leukemia (AML). JCO 38:12000–12000. https://doi.org/10.1200/JCO.2020.38.15_suppl.12000
Funding
This study was funded by the Conquer Cancer—Harry F. Bisel, MD, Endowed Young Investigator Award from the American Society of Clinical Oncology (DRR). DRR was also supported by an award from the NIH/NCI, 1L30CA264755-01, during the time of the research.
Author information
Authors and Affiliations
Contributions
Conception and design: DRR, RT, WAW. Data analysis: DRR, CJM, RT. Data review: DRR, CJM, RT. Manuscript preparation: All authors.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the UNC Lineberger Comprehensive Cancer Center Protocol Review Committee and the University of North Carolina IRB.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Richardson, D.R., Mhina, C.J., Teal, R. et al. Experiences of treatment decision-making among older newly diagnosed adults with acute myeloid leukemia: a qualitative descriptive study. Support Care Cancer 32, 197 (2024). https://doi.org/10.1007/s00520-024-08397-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08397-3


