Abstract
Purpose
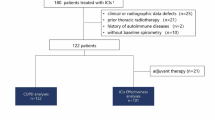
Immune checkpoint inhibitors (ICI) have become standard of care for some types of lung cancer. Along with expanding usage comes the emergence of immune-related adverse events (irAEs), including ICI-related pneumonitis (ICI-P). Treatment guidelines for managing irAEs have been developed; however, how clinicians manage irAEs in the real-world setting is less well known. We aimed to describe the outcomes and care patterns of grade ≥ 3 ICI-P in an onco-hospitalist service.
Patients and methods
We included patients with lung cancer treated with ICI who were admitted to an oncology hospitalist service with a suspicion of ICI-P. We described the hospitalization characteristics, treatment patterns, discharge practices, and clinical outcomes of patients with confirmed ICI-P. The primary outcome was time to start treatment for ICI-P.
Results
Among 49 patients admitted with a suspicion of ICI-P, 31 patients were confirmed to have ICI-P and subsequently received ICI-P directed treatment. Pulmonology was consulted in 97% of patients. Median time to start treatment for ICI-P was 1 day (IQR 0–3.5 days). All 31 patients received corticosteroids. Inpatient mortality was 32%. Majority of patients discharged with steroids were prescribed prophylaxis for gastritis and opportunistic infections. Thirty-eight percent of patients were seen by pulmonology and 86% were seen by the oncology team post-discharge.
Conclusion
Our study confirms prior findings of high mortality among patients with high-grade ICI-P. Early diagnosis and treatment are key to improving clinical outcomes. Understanding the care patterns and adherence to treatment guidelines of clinicians caring for this patient population may help identify ways to further standardize management practices and improve patient outcomes.

Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Zimmermann S et al (2018) Immune checkpoint inhibitors in the management of lung cancer. Am Soc Clin Oncol Educ Book 38:682–695
Alexander W (2016) The checkpoint immunotherapy revolution: what started as a trickle has become a flood, despite some daunting adverse effects; new drugs, indications, and combinations continue to emerge. P T 41(3):185–191
Horn L et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229
Suazo-Zepeda E et al (2021) Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Immunol Immunother 70(11):3069–3080
NCCN. National Comprehensive Cancer Network Guidelines in Oncology: management of immunotherapy-related toxicities. 2022 February 10, 2023]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
Shannon VR (2020) Pneumonitis associated with immune checkpoint inhibitors among patients with non-small cell lung cancer. Curr Opin Pulm Med 26(4):326–340
Wang DY et al (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4(12):1721–1728
Tiu BC et al (2022) Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J Immunother Cancer 10:6
Haanen J et al (2022) Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Ann Oncol 33(12):1217–1238
MASCC.org. 2020 clinical practice recommendations for the management of immune-mediated adverse events from checkpoint inhibitors. 2020 [cited 2023; Available from: https://mascc.org/2020-clinical-practice-recommendations-for-the-management-of-immune-mediated-adverse-events-from-checkpoint-inhibitors/
Sears CR et al (2019) Knowledge gaps and research priorities in immune checkpoint inhibitor-related pneumonitis. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 200(6):e31–e43
Zhong L et al (2020) Immune-related adverse events: pneumonitis. Adv Exp Med Biol 1244:255–269
Brahmer JR et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36(17):1714–1768
Manzano JG et al (2019) Demonstrating value: association of cost and quality outcomes with implementation of a value-driven oncology-hospitalist inpatient collaboration for patients with lung cancer. BMJ Open Qual 8(1):e000381
Dafni U et al (2019) Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 134:127–140
Gubens MA, Davies M (2019) NCCN guidelines updates: new immunotherapy strategies for improving outcomes in non-small cell lung cancer. J Natl Compr Canc Netw 17(5.5):574–578
Pang L et al (2023) Clinical characteristics and therapeutic effects of checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. BMC Cancer 23(1):203
Suresh K et al (2019) Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol 14(3):494–502
Atchley WT et al (2021) Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest 160(2):731–742
Naidoo J et al (2017) Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 35(7):709–717
Balaji A et al (2019) Immune-related adverse events requiring hospitalization: spectrum of toxicity, treatment, and outcomes. J Oncol Pract 15(9):e825–e834
Naidoo J et al (2019) A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J Natl Compr Canc Netw 17(6):712–720
Smithy JW, Faleck DM, Postow MA (2022) Facts and hopes in prediction, diagnosis, and treatment of immune-related adverse events. Clin Cancer Res 28(7):1250–1257
Funding
Research at The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health/NCI Cancer Center Support Grant, P30CA016672.
Author information
Authors and Affiliations
Contributions
Study concept and design: Joanna-Grace M Manzano MD MPH, Jeffrey Aldrich MD, Maggie Lu PharmD, Mahran Shoukier MD, Norman Brito-Dellan MD.
Data collection: Joanna-Grace M Manzano MD MPH, Hadeel Sahar MD, Jeffrey Aldrich MD, Maggie Lu PharmD, Mahran Shoukier MD, Kodwo Dickson MD, Kwame Koom-Dadzie MD, Ed Kheder MD, Maria Franco-Vega MD, Alyssa Mohammed MD, Mayoora Muthu DO, Cesar Simbaqueba MD, Michelle Sibille Senechalle MD, Norman Brito-Dellan MD.
Data analysis and interpretation: Joanna-Grace M Manzano MD MPH, Hadeel Sahar MD, Jeffrey Aldrich MD, Maggie Lu PharmD, Mahran Shoukier MD, Norman Brito-Dellan MD.
Statistical analysis and interpretation: Christine B Peterson PhD, Joanna-Grace M Manzano MD MPH.
Manuscript preparation and final approval: Joanna-Grace M Manzano MD MPH, Hadeel Sahar MD, Jeffrey Aldrich MD, Maggie Lu PharmD, Mahran Shoukier MD, Christine B Peterson PhD, Kodwo Dickson MD, Kwame Koom-Dadzie MD, Ed Kheder MD, Maria Franco-Vega MD, Alyssa Mohammed MD, Mayoora Muthu DO, Cesar Simbaqueba MD, Michelle Sibille Senechalle MD, Norman Brito-Dellan MD.
Corresponding author
Ethics declarations
Ethics approval
The Quality Improvement Assessment Board at our institution approved this study. Ethical approval was not required by the reviewing/approving body.
Consent to participate
Not applicable.
Consent for publication
Not applicable. There is no patient identifiable data in this publication.
Competing interests
Maggie Lu has the following declarations outside the submitted work holds stock with Amgen. The authors have no other relevant disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manzano, JG.M., Sahar, H., Aldrich, J. et al. Treatment patterns and outcomes of high-grade immune checkpoint inhibitor-related pneumonitis in an oncology hospitalist service. Support Care Cancer 32, 160 (2024). https://doi.org/10.1007/s00520-024-08361-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08361-1