Abstract
Purpose
Neutropenic fever remains a major complication in acute leukemia. Decolonization is assumed as a promising intervention for eradicating causative agents of infection.
Methods
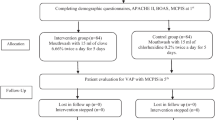
In this randomized clinical trial, 96 patients with acute leukemia were assigned randomly to mupirocin nasal drop 2% (n = 32), chlorhexidine mouthwash 0.2% (n = 33), and control group (n = 31). In control group, patients did not receive any medication for decolonization. All patients received treatment for 5 days (2 days prior to chemotherapy until 3 days after chemotherapy). Pharynx and nasal swabs were taken prior to the intervention and at the end of decolonization period in all groups. Antibiotic susceptibility testing was performed by the disc diffusion method in order to identify bacterial isolates.
Results
Bacterial recovery of both nasal and pharynx swabs was observed after global decolonization with mupirocin nasal drop. Decolonization with mupirocin significantly eradicated Coagulase-negative staphylococci (CONS) in both nasal and pharynx swabs (p-value = 0.000). Moreover, mupirocin decreased Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) species. Chlorhexidine mouthwash significantly eradicated CONS in pharynx swabs (p-value = 0.000). In addition, both decolonization strategies decreased both antibiotic use and frequency of fever in leukemic patients.
Conclusion
Global decolonization with mupirocin nasal drop not only eradicates both nasal and pharynx microorganisms, but also reduces antibiotic requirement and frequency of fever in patients with acute leukemia.
The protocol of the present study was approved on December 2016 (registry number: IRCT20160310026998N6).

Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to information that could compromise the privacy of research participants.
References
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol 90(3):190–199. https://doi.org/10.1016/j.critrevonc.2013.12.006
Lucas AJ, Olin JL, Coleman MD (2018) Management and preventive measures for febrile neutropenia. P t 43(4):228–232
Mullen CA (2003) Ciprofloxacin in treatment of fever and neutropenia in pediatric cancer patients. Pediatr Infect Dis J 22(12):1138–1142. https://doi.org/10.1097/01.inf.0000101993.83884.ed
Segal BH, Freifeld AG, Baden LR, Brown AE, Casper C, Dubberke E et al (2008) Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 6(2):122–174. https://doi.org/10.6004/jnccn.2008.0013
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA et al (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52(4):e56-93. https://doi.org/10.1093/cid/cir073
Sakr A, Brégeon F, Rolain JM, Blin O (2019) Staphylococcus aureus nasal decolonization strategies: a review. Expert Rev Anti Infect Ther 17(5):327–340. https://doi.org/10.1080/14787210.2019.1604220
Septimus EJ, Schweizer ML (2016) Decolonization in prevention of health care-associated infections. Clin Microbiol Rev 29(2):201–222. https://doi.org/10.1128/cmr.00049-15
Tucaliuc A, Blaga AC, Galaction AI, Cascaval D (2019) Mupirocin: applications and production. Biotechnol Lett 41(4–5):495–502. https://doi.org/10.1007/s10529-019-02670-w
Jones CG (2000) Chlorhexidine: is it still the gold standard? Periodontol 1997(15):55–62. https://doi.org/10.1111/j.1600-0757.1997.tb00105.x
Adhikari R, Pant ND, Neupane S, Neupane M, Bhattarai R, Bhatta S, Chaudhary R, Lekhak B (2017) Detection of methicillin resistant staphylococcus aureus and determination of minimum inhibitory concentration of vancomycin for staphylococcus aureus isolated from pus/wound swab samples of the Patients Attending a Tertiary Care Hospital in Kathmandu, Nepal. Can J Infect Dis Med Microbiol 2017:6. https://doi.org/10.1155/2017/2191532
Guidelines in the management of febrile neutropenia for clinical practice. Supplements and Featured Publications 2017, https://www.ajmc.com/view/guidelines-in-the-management-of-febrile-neutropenia-for-clinical-practice
Mohammadzadeh M, Farashi E, Hesam AR, Chavoshi SH, Ghaffary S (2021) Drug utilization evaluation of agents administered for prevention and treatment of cancer-related infections. J Pharm Care 9(3):119–128
De Rosa FG, Motta I, Audisio E, Frairia C, Busca A, Di Perri G et al (2013) Epidemiology of bloodstream infections in patients with acute myeloid leukemia undergoing levofloxacin prophylaxis. BMC Infect Dis 13:563. https://doi.org/10.1186/1471-2334-13-563
Kamboj M, Sepkowitz KA (2009) Nosocomial infections in patients with cancer. Lancet Oncol 10(6):589–597. https://doi.org/10.1016/s1470-2045(09)70069-5
Tag ElDein MA, Yassin AS, El-Tayeb O, Kashef MT (2021) Chlorhexidine leads to the evolution of antibiotic-resistant Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 40(11):2349–2361. https://doi.org/10.1007/s10096-021-04292-5
Nakahara H, Kozukue H (1982) Isolation of chlorhexidine-resistant Pseudomonas aeruginosa from clinical lesions. J Clin Microbiol 15(1):166–168. https://doi.org/10.1128/jcm.15.1.166-168.1982
Büyükkoçak Ü, Ağalar C, Deniz T, Çeken S, Ağalar F (2015) Effect of chlorhexidine on oral airway biofilm formation of Staphylococcus epidermidis. J Microbiol Infect Dis 5(4):162–166
Schmidt K, Estes C, McLaren A, Spangehl MJ (2018) Chlorhexidine antiseptic irrigation eradicates Staphylococcus epidermidis from biofilm: an in vitro study. Clin Orthop Relat Res 476(3):648
Climo MW, Sepkowitz KA, Zuccotti G, Fraser VJ, Warren DK, Perl TM, Speck K, Jernigan JA, Robles JR, Wong ES (2009) The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 37(6):1858–1865. https://doi.org/10.1097/CCM.0b013e31819ffe6d
La Combe B, Mahérault AC, Messika J, Billard-Pomares T, Branger C, Landraud L et al (2018) Oropharyngeal bacterial colonization after chlorhexidine mouthwash in mechanically ventilated critically Ill patients. Anesthesiology 129(6):1140–1148. https://doi.org/10.1097/aln.0000000000002451
Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani M (2017) The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults’ intensive care units. J Crit Care 40:318–22. https://doi.org/10.1016/j.jcrc.2017.02.029
Buehlmann M, Frei R, Fenner L, Dangel M, Fluckiger U, Widmer AF (2008) Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol 29(6):510–516. https://doi.org/10.1086/588201
Organization GWH. Global guidelines for the prevention of surgical site infection web appendix 3. Summary of a systematic review on decolonization with mupirocin ointment with or without chlorhexidine gluconate body wash for the prevention of Staphylococcus aureus infection in nasal carriers undergoing surgery. 2018, https://www.ncbi.nlm.nih.gov/books/NBK536421/?report=classic#_ncbi_dlg_citbx_NBK536421
Mody L, Kauffman CA, McNeil SA, Galecki AT, Bradley SF (2003) Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 37(11):1467–1474. https://doi.org/10.1086/379325
Ishikawa J, Horii T (2005) Effects of mupirocin at subinhibitory concentrations on biofilm formation in Pseudomonas aeruginosa. Chemotherapy 51(6):361–362. https://doi.org/10.1159/000088962
Horii T, Morita M, Muramatsu H, Muranaka Y, Kanno T, Maekawa M (2003) Effects of mupirocin at subinhibitory concentrations on flagella formation in Pseudomonas aeruginosa and Proteus mirabilis. J Antimicrob Chemother 51(5):1175–1179. https://doi.org/10.1093/jac/dkg226
Eed EM, Ghonaim MM, Khalifa AS, Alzahrani KJ, Alsharif KF, Taha AA (2019) Prevalence of mupirocin and chlorhexidine resistance among methicillin-resistant coagulase-negative staphylococci isolated during methicillin-resistant Staphylococcus aureus decolonization strategies. Am J Infect Control 47(11):1319–1323. https://doi.org/10.1016/j.ajic.2019.05.004
Acknowledgements
We would like to acknowledge all the nurses and staffs of Shahid Ghazi Hospital for collaboration with this research during the study.
Author information
Authors and Affiliations
Contributions
Saba Ghaffary: Conceptualization, data curation, validation, investigation, visualization, methodology, supervision, writing–original draft and editing. Aref Javidnia: Data Collection, investigation, writing. Samineh Beheshtirouy: Conceptualization, writing–original draft, writing–review and editing. Javid Sadeghi: Resources, supervision, validation, investigation, methodology. Aliakbar Movassaghpour Akbari: Conceptualization, resources, validation, methodology, project administration. Hamed Hamishehkar: Resources, validation, methodology. Parvin Sarbakhsh: Methodology, analysis, writing–original draft and editing. Alireza Nikanfar: Validation, Data Collection. Zohreh Sanaat: Validation, Data Collection. Ali Esfahani: Validation, Data Collection. Seyed Hadi Chavoshi: Validation, Data Collection. Babak Nejati: Validation, Data Collection. Mortaza Raeisi: Validation, Data Collection. Nasrin Gholami: Validation, Data Collection.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The protocol of the present study was approved by the local Ethics Committee of Tabriz University of Medical Sciences, Iran on December 2016. The trial was registered in the Iranian Registry of Clinical Trials (registry number: IRCT20160310026998N6).
Consent to participate
All patients were informed about the trial and gave a written informed consent before the study initiation.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghaffary, S., Javidnia, A., Beheshtirouy, S. et al. Comparison of global decolonization efficacy with mupirocin nasal drop and chlorhexidine mouthwash in acute leukemia patients: randomized clinical trial. Support Care Cancer 32, 42 (2024). https://doi.org/10.1007/s00520-023-08232-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08232-1