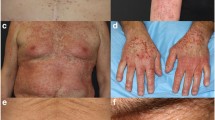
Abstract
Purpose
Dermatologic adverse events commonly result in the interruption of oncologic treatment, and targeted therapies are the most frequently interrupted class of anticancer agents. Alopecia is a common cutaneous adverse event reported with CK4/6i therapy. Though the clinical characteristics and therapeutic response of EIA have been well documented, few studies have characterized alopecia in patients treated with CDK4/6i.
Methods
This study analyzed a retrospective cohort of 28 breast cancer patients diagnosed with endocrine-induced alopecia (EIA) or CDKiA. Comparative analysis of the clinical characteristics of alopecia and therapeutic response to minoxidil was conducted. Therapeutic response to minoxidil (LDOM or topical [5%] solution or foam) was assessed by both Dean Scale and qualitative clinical improvement by comparison of pretreatment and posttreatment clinical images by single-blinded, board-certified academic dermatologists (ST and BD).
Results
CDKiA was clinically similar to androgenetic alopecia and specific vertex involvement was more common in patients treated with CDK4/6i + ET than endocrine monotherapy (n = 7 [70.0%] vs n = 4 [36.4%]; p = 0.04), respectively. After 4–6 months of minoxidil, there was a moderate to significant qualitative alopecia improvement in 80% of CDKiA patients versus 94.4% of EIA patients. Additionally, superior improvement of mean Dean Score grade was observed in EIA (with change from pre- to posttreatment − 0.44; p = 0.0002).
Conclusion
Compared to endocrine monotherapy, patients on combination CDK4/6i + ET had greater extent of vertex involvement and were more recalcitrant to minoxidil. The preferential vertex involvement observed in CDKiA suggests that combination therapy with minoxidil and topical antiandrogens with poor systemic absorption should be studied in this setting.



Similar content being viewed by others
Data Availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- EIA:
-
Endocrine therapy-induced alopecia
- CDK4/6i:
-
Cyclin-dependent kinase 4 and 6 inhibitors
- CDKiA:
-
CDK4/6i-induced alopecia
- BC:
-
Breast cancer
- CIA:
-
Chemotherapy-induced alopecia
References
Eiger D, Wagner M, Pondé NF, Nogueira MS et al (2020) The impact of cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) on the incidence of alopecia in patients with metastatic breast cancer (BC). Acta Oncol 59(6):723–725
Beusterien K, Maculaitis MC, Hallissey B et al (2021) Patient, oncologist, and payer preferences for adjuvant endocrine therapy and CDK4/6 inhibitor regimens in early-stage breast cancer: a discrete choice experiment. Patient Prefer Adherence 15:611–623. https://doi.org/10.2147/PPA.S298670
Saggar V, Wu S, Dickler MN, Lacouture ME (2013) Alopecia with endocrine therapies in patients with cancer. Oncologist 18(10):1126–1134. https://doi.org/10.1634/theoncologist.2013-0193
Silvestri M, Cristaudo A, Morrone A et al (2021) Emerging skin toxicities in patients with breast cancer treated with new cyclin-dependent kinase 4/6 inhibitors: a systematic review. Drug Saf 44(7):725–732
Chan D, Freites Martinez AD, Goldfarb SB et al (2019) CDK4/6 plus aromatase inhibitor-induced alopecia in breast cancer patients. J Clin Oncol 37(15_suppl):e 12537. https://doi.org/10.1200/JCO.2019.37.15_suppl.e12537
Finn RS, Crown JP, Lang I et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Cristofanilli M, Turner NC, Bondarenko I et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17:425–439
Slamon DJ, Neven P, Chia S et al (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36(24):2465–2472
Tripathy D, Im SA, Colleoni M et al (2018) Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 19(7):904–915
Sledge GW, Toi M, Neven P et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884
Goetz MP, Toi M, Campone M et al (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35(32):3638–3646
Lee WS, Ro BI, Hong SP, Bak H et al (2007) A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol 57(1):37–46
Freites-Martinez A, Shapiro J, Chan D et al (2018) Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol 154(6):670–675
Chawla S, Hill A, Fearfield L, Johnston S, Parton M, Heelan K (2021) Cutaneous toxicities occurring during palbociclib (CDK4/6 inhibitor) and endocrine therapy in patients with advanced breast cancer: a single-centre experience. Breast Cancer Res Treat 188(2):535–545
Raschi E, Fusaroli M, La Placa M et al (2022) Skin toxicities with cyclin-dependent kinase 4/6 inhibitors in breast cancer: signals from disproportionality analysis of the FDA adverse event reporting system. Am J Clin Dermatol 23(2):247–255
Sollena P, Vasiliki N, Kotteas E, Stratigos AJ et al (2023) On behalf of the EADV Task Force Dermatology For Cancer Patients. Cyclin-dependent kinase 4/6 inhibitors and dermatologic adverse events: results from the EADV task force “Dermatology for Cancer Patients” International Study. Cancers (Basel) 15(14):3658
Barrios DM, Phillips GS, Freites-Martinez A et al (2020) Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study. J Eur Acad Dermatol Venereol 34:1340–1347
Freites-Martinez A, Shapiro J, Goldfarb S et al (2019) Hair disorders in patients with cancer. J Am Acad Dermatol 80(5):1179–1196
Rossi A, Caro G, Magri F, Fortuna MC, Carlesimo M (2021) Clinical aspect, pathogenesis and therapy options of alopecia induced by hormonal therapy for breast cancer. Explor Target Antitumor Ther 2(5):490–495
Kang J, Lee JW, Kwon O (2023) Efficacy of low-dose oral minoxidil in the management of anticancer therapy-induced alopecia in patients with breast cancer: a retrospective cohort study. J Am Acad Dermatol 88(5):1170–1173
Purba TS, Ng’andu K, Brunken L, Smart E, Mitchell E, Hassan N, O’Brien A, Mellor C, Jackson J, Shahmalak A, Paus R (2019) CDK4/6 inhibition mitigates stem cell damage in a novel model for taxane-induced alopecia. EMBO Mol Med 11(10):e11031
York K, Meah N, Bhoyrul B, Sinclair R (2020) A review of the treatment of male pattern hair loss. Expert Opin Pharmacother 21(5):603–612
Wei C, Bovonratwet P, Gu A, Moawad G et al (2020) Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol 83(4):1021–1027
Rozner RN, Freites-Martinez A, Shapiro J et al (2019) Safety of 5α-reductase inhibitors and spironolactone in breast cancer patients receiving endocrine therapies. Breast Cancer Res Treat 174(1):15–26
Gupta Aditya K, Talukder Mesbah et al (2022) Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J Dermatol Treat 33(7):2946–2962. https://doi.org/10.1080/09546634.2022.2109567
Wang C, Du Y, Bi L, Lin X, Zhao M, Fan W (2023) The efficacy and safety of oral and topical spironolactone in androgenetic alopecia treatment: a systematic review. Clin Cosmet Investig Dermatol 9(16):603–612. https://doi.org/10.2147/CCID.S398950.PMID:36923692;PMCID:PMC10010138
Abdel-Raouf H, Aly UF, Medhat W et al (2021) A novel topical combination of minoxidil and spironolactone for androgenetic alopecia: clinical, histopathological, and physicochemical study. Dermatol Ther 34(1):e14678
Souza A, Strober BE (2012) Chapter 214. Principles of topical therapy. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K (eds) Fitzpatrick's Dermatology in General Medicine, 8e. McGraw Hill. https://accessmedicine.mhmedical.com/content.aspx?bookid=392§ionid=41138950
Mackenzie IS, Macdonald TM, Thompson A et al (2012) Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ 13(345):e4447
Alvarez A, Bernal AM, Anampa J (2023) Racial disparities in overall survival after the introduction of cyclin-dependent kinase 4/6 inhibitors for patients with hormone receptor-positive, HER2-negative metastatic breast cancer. Breast Cancer Res Treat 198(1):75–88
Messina C, Cattrini C, Buzzatti G et al (2018) CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat 172:9–21
Funding
AM is supported by the Pelotonia Scholars Program, and BD is supported by a Dermatology Foundation Career Development Award.
Author information
Authors and Affiliations
Contributions
The initial draft of the manuscript was written by Abena Minta and Lucy Rose, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ohio State University Institutional Review Board (IRB#2021H0264).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Loprinzi reports personal fees from PledPharma, personal fees from Disarm Therapeutics, personal fees from Asahi Kasei/Veloxis, personal fees from Metys Pharmaceuticals, personal fees from OnQuality, personal fees from Mitsubishi Tanabe, personal fees from NKMax, personal fees from Novartis, personal fees from HengRui, personal fees from Nuro Bio, personal fees from Osmol Therapeutics, Inc., personal fees from Grunenthal, personal fees from Genentech, personal fees from Bexion, personal fees from Emmes Company, personal fees from Pfizer, and personal fees from Toray, all outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Minta, A., Rose, L., Park, C. et al. Retrospective cohort study of CDK4/6-inhibitor-induced alopecia in breast cancer patients. Support Care Cancer 31, 717 (2023). https://doi.org/10.1007/s00520-023-08160-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08160-0