Abstract
Purpose
Docetaxel + cisplatin + 5-fluorouracil (DCF) therapy, a frequently prescribed regimen for esophageal cancer, is associated with a high risk of febrile neutropenia (FN). This study investigated whether a low skeletal muscle mass index (SMI) is an independent risk factor for FN.
Methods
This retrospective, observational study investigated the SMI of patients with esophageal cancer who received DCF therapy between March 2018 and July 2020. Based on the Asian sarcopenia criteria, patients were divided into two groups: high and low SMI (SMI of < 7.0 and 5.7 kg/m2 for males and females, respectively). The incidence of FN was then compared between the two groups.
Results
Thirty-nine patients (20 and 19 in the high- and low-SMI groups, respectively) were included in this study. The incidence of FN was significantly higher in the low-SMI group (63.2% vs. 20.0%, P = 0.006). Univariable and multivariable logistic regression analyses revealed that a low SMI was an independent risk factor for FN (odds ratio, 7.178; 95% confidence interval, 1.272–40.507; P = 0.026). In addition, the frequency of dose reduction in DCF therapy was significantly higher in the low-SMI group (68.4% vs. 35.0%, P = 0.037).
Conclusion
Low SMI is an independent risk factor for FN in patients with esophageal cancer receiving DCF therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is highly metastatic and has a poor prognosis. Moreover, esophageal cancer is susceptible to lymph node metastasis even in the early stages owing to high-lymph fluid flow through the esophagus [1]. Preoperative chemo(radio)therapy has been adopted worldwide to treat advanced esophageal cancer (stage II/III). In Western countries, chemoradiotherapy (chemotherapy + radiation) is commonly employed as a preoperative therapy [2, 3], whereas chemotherapy alone is widely used in Japan [4]. The cisplatin (CDDP) + 5-fluorouracil (5-FU) therapy (FP therapy) has been recommended and employed as preoperative chemotherapy in Japan. However, owing to its poor 5-year survival rate (54.5%) [5], docetaxel (DOC) + CDDP + 5-FU therapy (DCF therapy) has been introduced as an alternative to FP therapy in recent years. Besides its preferable efficacy, DCF therapy is associated with a high risk of adverse reactions [6, 7]. Among the various adverse reactions, febrile neutropenia (FN) is observed at high frequencies (approximately 20–40%) without prophylactic antibiotics and/or granulocyte-colony stimulating factor with primary prophylaxis [8,9,10,11,12,13]. Although the current guidelines do not uniformly recommend the prophylactic administration of pegfilgrastim (PEG-G) to patients receiving DCF therapy, several reports have suggested the efficacy of prophylactic PEG-G during DCF therapy [13,14,15]. FN is associated with a decrease in the relative dose intensity (RDI) and subsequent poor prognosis [16,17,18,19]. Therefore, risk factors for FN during DCF therapy have been extensively studied, and age, solitary life, and non-use of PEG-G have been identified as risk factors for FN [15, 20]. However, not all patients with these reported risk factors develop FN, suggesting that additional risk factors need to be explored.
Sarcopenia, defined as “age-related loss of skeletal muscle mass (SMM) with loss of muscle strength and/or reduced physical performance” [21], has been gaining attention as a predictive factor for the efficacy and safety of chemotherapy [22,23,24]. Patients with esophageal cancer are at a high risk of sarcopenia because malnutrition due to the loss of oral food intake is often observed [25]. Although several studies have investigated the impact of sarcopenia (loss of SMM) on the safety and efficacy of DCF therapy in patients with esophageal cancer [26, 27], the results are controversial. For instance, Kamitani et al. [26] reported that SMM loss is associated with worsening of overall survival (OS) and disease-specific survival (DSS) in patients treated with DCF therapy, whereas Miyata et al. [27] reported that low SMM was not a significant risk factor for developing FN. In recent years, the SMM index (SMI), calculated by dividing appendicular SMM by the square of height, has been adopted as one of the criteria for sarcopenia in the latest version of the Asian Working Group for Sarcopenia 2019 (AWGS 2019) diagnostic criteria [21], and SMI of 7.0 kg/m2 (5.7 kg/m2 for female) is recommended as the cutoff value. Considering these circumstances, SMI is expected to be widely used in clinical settings. However, to the best of our knowledge, whether SMI is associated with an increased risk of FN has not been elucidated.
This study is aimed at assessing the impact of SMI measured before DCF therapy on FN risk. Although dual-energy X-ray absorptiometry (DEXA) or computed tomography (CT) has been used as the gold standard for measuring SMI in previous studies, we employed bioelectrical impedance analysis (BIA) to measure SMI because of the simplicity of the measurement. SMI measured by BIA is reportedly favorably consistent with that measured using DEXA or CT [28,29,30]. Furthermore, the latest version of the AWGS 2019 diagnostic criteria [21] adopts BIA as a method for measuring SMI along with DEXA.
Patients and methods
Patients
This study included patients with esophageal cancer who received DCF therapy and whose SMI was measured at The University of Tokyo Hospital between March 2018 and July 2020. The exclusion criteria were as follows: (1) patients who were enrolled in other prospective, interventional clinical trials; (2) patients who started chemotherapy > 45 days after the last SMI measurement; (3) patients whose CDDP dosage was lower than the appropriately adjusted dosage depending on the creatinine clearance (Ccr) calculated using the Cockcroft and Gault equation [31] (30 mL/min ≤ Ccr < 40 mL/min, 35 mg/m2; 40 mL/min ≤ Ccr < 50 mL/min, 50 mg/m2; 50 mL/min ≤ Ccr < 60 mL/min, 60 mg/m2; 60 mL/min ≤ Ccr, 70 mg/m2); and (4) patients whose chemotherapy was discontinued for reasons other than adverse reactions related to DCF therapy.
Treatment and supportive care
DCF therapy consisted of intravenous infusion of DOC (70 mg/m2) for 1 h, followed by intravenous infusion of CDDP (70 mg/m2; dosage was adjusted according to Ccr) for 2 h on day 1, and continuous intravenous infusion of 5-FU (700 mg/m2) from days 1 to 5. In this study, one cycle of 21 days or 28 days was adopted for the purpose of neoadjuvant chemotherapy (NAC) or other purposes (e.g., treatment for unresectable/recurrent esophageal cancer, hereafter referred to as “non-NAC”), respectively. Oral levofloxacin (500 mg; dosage adjusted according to Ccr) was administered to all patients from days 5 to 15 as prophylaxis for FN. MgSO4 (20 mEq) was administered before CDDP administration, and adequate hydration with normal saline was administered [32], to avoid CDDP-induced renal dysfunction. In our institute, PEG-G is administered on day 7 of DCF therapy because the G-CSF guideline [33] recommends administering it 24 h after anticancer drug administration.
The antiemetic agents used were neurokinin 1 (NK1) receptor antagonist ((fos)aprepitant), serotonin (5-hydroxytryptamine)-3 (5-HT3) receptor antagonist (palonosetron), and dexamethasone [34]. The patients received oral aprepitant (125 mg) or intravenous fosaprepitant (150 mg) on day 1. Intravenous palonosetron (0.75 mg) and dexamethasone (9.9 mg) were administered on day 1, followed by intravenous dexamethasone (6.6 mg) on days 2–5. The patients also received oral aprepitant (80 mg) on days 2 and 3 when they received oral aprepitant on day 1.
Data collection and definition
The database comprised the following patient characteristics: age, sex, height, body weight (BW), body mass index (BMI), the purpose of chemotherapy, family structure, distant metastasis, supportive care, reported FN risk factors, laboratory data, adverse reactions, and dose reduction/discontinuation. FN was defined as (1) an axillary temperature of ≥ 37.5 °C and an absolute neutrophil count (ANC) of < 500 cells/µL, or (2) an axillary temperature of ≥ 37.5 °C and an ANC of < 1000 cells/µL with an expected decline to < 500 cells/µL or less within 48 h, according to practical clinical guidelines [35]. In this study, we diagnosed patients with an axillary temperature of ≥ 37.5 °C and an ANC of 500–1000 cells/µL as having FN only after we had confirmed that the attending physician had made an FN diagnosis. Other adverse reactions were evaluated using CTCAE version 5.0.
Measurement of skeletal muscle mass index
The body composition parameters of the patients (SMM, appendicular SMM, and lean body mass (LBM)) were measured before the initial DCF therapy using BIA (InBody 770 body composition analyzer, Biospace, Tokyo, Japan). SMI was calculated by dividing the appendicular SMM by the square of height. According to the AWGS 2019 criteria [21], the patients were classified into two groups: high-SMI (SMI ≥ 7.0 kg/m2 for males or SMI ≥ 5.7 kg/m2 for females) group and low-SMI (SMI < 7.0 kg/m2 for males or SMI < 5.7 kg/m2 for females).
Statistical analysis
Continuous data are expressed as median (range) unless otherwise mentioned. The Mann–Whitney U test was used to compare continuous variables, while the chi-square test was used to analyze categorical data. Fisher’s exact test was used as a replacement for the chi-square test when the expected frequency of one or more cells was < 5. Univariable and multivariable logistic regression analyses were conducted using age (≥ 65 or < 65 years), sex (female or male), purpose of DCF therapy (NAC or non-NAC), Ccr (< 50 mL/min or ≥ 50 mL/min), SMI (low or high), and prophylactic pegfilgrastim (non-user or user) to identify risk factors for developing FN. All tests were two-tailed, and P values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS Statistics for Windows, version 24 (Armonk, NY, IBM Corp.).
Results
Background characteristics of patients
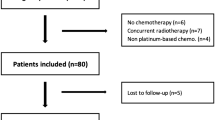
Among the 122 patients who received DCF therapy during the study period, SMI data were available for 96. Fifty-seven patients were excluded, and 39 were included in the analysis. As shown in Fig. 1, 20 and 19 patients were classified into the high- and low-SMI groups, respectively.
Table 1 shows the patients’ backgrounds, laboratory data, body composition parameters, and FN risk factors before DCF therapy. As expected, the body composition parameters (SMM, SMI, and LBM) were significantly lower in the low-SMI group than those in the high-SMI group. In addition, compared to the high-SMI group, the height, BW, BMI, serum albumin (Alb), and hemoglobin (Hb) were significantly lower in the low-SMI group. For other data, no statistically significant differences were observed between the two groups.
Adverse reactions, dose reduction/discontinuation
Table 2 shows the incidence of adverse reactions to DCF therapy. In this study, none of the patients developed FN grade 4 severity or greater. Compared with the high-SMI group, FN grade 3 (20.0% vs. 63.2%; P = 0.006), neutropenia grade 4 (35.0% vs. 73.7%; P = 0.015), and anorexia ≥ grade 3 (0.0% vs. 21.1%; P = 0.049) were significantly more frequently observed in the low-SMI group. Anorexia ≥ grade 2, nausea, and diarrhea tended to occur slightly more frequently in the low-SMI group, although there were no statistically significant differences between the two groups. Among the 16 patients in this study who developed FN grade 3, 13 (81.3%) developed FN during the first course, whereas the other three patients developed FN during the second or third course.
As FN, a dose-limiting toxicity of DCF therapy, was more frequently observed in the low-SMI group, we compared the proportion of patients whose dose was reduced or discontinued between the high- and low-SMI groups. As shown in Table 3, the proportion of patients who required dose reduction was significantly higher in the low-SMI group (35.0% vs. 68.4%; P = 0.037).
Univariable and multivariable logistic regression analyses
Because patients in the low-SMI group had a significantly higher FN risk, univariable and multivariable logistic regression analyses were performed to assess whether a low SMI was an independent risk factor for FN. As shown in Table 4, univariable and multivariable logistic regression analyses using six factors (age, sex, purpose of chemotherapy, Ccr, SMI, and prophylactic PEG-G use) as covariates revealed that only low SMI was an independent risk factor for FN (odds ratio, 7.178; 95% confidence interval, 1.272–40.507; P = 0.026).
Discussion
The results of this study revealed that low SMI is an independent risk factor for FN in patients with esophageal cancer receiving DCF therapy. In addition, it was also revealed that patients with low SMI are at a higher risk of dose reduction due to adverse reactions related to DCF therapy. To the best of our knowledge, this is the first study to identify low SMI as an independent risk factor for FN in patients with esophageal cancer receiving DCF therapy. We believe that SMI could be useful in predicting the individual risk of FN during DCF therapy in a clinical setting.
As shown in Table 2, 41.0% of the patients (16/39) developed FN, even though 53.8% received PEG-G as primary prophylaxis for FN. In addition, a statistically significant reduction in the incidence of FN was observed in patients receiving prophylactic PEG-G administration (61.1% (11/18) vs. 23.8% (5/21); relative risk reduction, 61%; P = 0.026, Table 2). Although a preventive effect of PEG-G was observed in our study, the magnitude of risk reduction was smaller than that in a previous study by Ohkura et al. [15] (3.0% vs. 32.2%; relative risk reduction, 91%). However, when stratified by the SMI of patients, the H-SMI group showed a similar magnitude of decrease in FN incidence (from 37.5 to 8.3%, Table 2) to that observed in the previous study. In contrast, the L-SMI group showed a more moderate decrease in FN incidence after prophylactic PEG-G administration (from 80 to 44%, Table 2). The distribution of SMI in the previous study is unknown, but it is possible that the proportion of patients with L-SMI status in our study was higher than that in the previous study by Ohkura et al. [15], and that this may have led to underestimation of the efficacy of prophylactic PEG-G. Furthermore, patients who received prior chemotherapy or who did not receive a full dose of DCF therapy because of organ dysfunction were excluded in the previous study by Ohkura et al., whereas our study included these patients, which may have increased the incidence of FN and decreased the prophylactic effect of PEG-G.
As shown in Table 2, the incidence of FN was significantly higher in the low-SMI group than that in the high-SMI group (63.2% vs. 20.0%, P = 0.006), and multivariate logistic regression analysis identified low SMI as an independent risk factor for FN development (odds ratio 7.178; P = 0.026) (Table 4). To the best of our knowledge, this study is the first to identify a low SMI as an independent risk factor for FN. Although the underlying mechanisms of this observed relationship between low SMI and increased risk of FN remain to be elucidated, there are several possible explanations. One is that a low SMI reflects low Alb, which is already known as a risk factor for FN [36, 37]. Second, low SMI is associated with alterations in the pharmacokinetics (PK) of chemotherapeutic agents. From this point of view, several previous reports have indicated that the body composition parameters of patients alter the PK of chemotherapeutic agents. For instance, Gusella et al. [38] reported that the clearance of 5-FU strongly correlates with fat-free mass (FFM) rather than BW and body surface area, and patients with lower FFM exhibited lower 5-FU clearance. In addition, several previous studies have revealed that the LBM-normalized dosage of CDDP and 5-FU was higher in patients with hematological toxicity than in those without hematological toxicity, suggesting that LBM is well correlated with 5-FU clearance [39,40,41]. Because it has been reported that FFM is roughly equal to LBM [42], and a strong correlation was observed between LBM and SMI in our study (rs = 0.872, P < 0.001, Supplementary Fig. S1 (Online Resource 1)), it is possible that the clearance of 5-FU decreased and its blood concentrations increased in the low-SMI group, and that the increased drug exposure was associated with an increased risk of adverse reactions. Future PK studies are required to fully elucidate the relationship between clearance of chemotherapeutic agents and SMI.
Because low SMI was identified as a risk factor for FN, we compared the proportion of patients who required dose reduction/discontinuation, as FN is a major dose-limiting toxicity during DCF therapy. As shown in Table 3, the proportion of patients with dose reduction was significantly higher in the low-SMI group than that in the high-SMI group (68.4% vs. 35.0%, P = 0.037). Considering that the RDI of DCF therapy has been reported to affect long-term prognosis in previous literature [17,18,19], the higher proportion of patients whose dose was reduced in the low-SMI group may have led to a worse long-term prognosis. Although the relationship between low SMI and long-term prognosis needs to be evaluated in future studies, normalizing SMI by improving nutritional status before DCF therapy may improve safety and long-term prognosis.
Although our study provided clinically important information, it had several limitations that should be considered. First, this was a single-center, retrospective, observational study with a relatively small number of patients; thus, it was impossible to completely exclude unintended biases that may have affected the results. Therefore, the results of this study need to be verified in future studies with larger sample sizes. Second, body composition parameters were measured using the BIA method only, and DEXA or CT, the gold standards for measuring body composition parameters, was not used in this study. Although data obtained by BIA correlate well with those obtained by DEXA or CT [28,29,30], it is uncertain whether our results can be generalized to SMI data measured by DEXA or CT. Third, this study did not investigate the long-term prognosis, such as OS and DSS. Further studies are needed to clarify the relationship between SMI and long-term prognosis. Fourth, the dose of 5-FU used in this study (700 mg/m2) was different from that used in the JCOG 1109 trial (750 mg/m2). Therefore, the generalizability of the results of this study to patients who receive 5-FU at a dose of 750 mg/m2 should be confirmed in the future studies.
In conclusion, we identified low SMI as an independent risk factor for FN in patients with esophageal cancer receiving DCF therapy. We also revealed that a low SMI was associated with a higher risk of dosage reduction during DCF therapy. Considering the simplicity of measuring SMI using the BIA method, SMI would be a useful predictive factor for FN, and SMI measurement before DCF therapy would increase the safety and efficacy of chemotherapy in patients with esophageal cancer scheduled to receive DCF therapy.
Data availability
The data supporting the findings of this study are available upon request from the corresponding author. The data were not publicly available because of privacy or ethical restrictions.
Code availability
Not applicable
References
Akutsu Y, Kato K, Igaki H et al (2016) The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann Surg 264:1009–1015. https://doi.org/10.1097/SLA.0000000000001557
Shah MA, Kennedy EB, Catenacci DV et al (2020) Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol 38:2677–2694. https://doi.org/10.1200/JCO.20.00866
Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D (2013) Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi51–vi56. https://doi.org/10.1093/annonc/mdt342
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 16:1–24. https://doi.org/10.1007/s10388-018-0641-9
Ando N, Kato H, Igaki H et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19:68–74. https://doi.org/10.1245/s10434-011-2049-9
Hara H, Tahara M, Daiko H et al (2013) Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 104:1455–1460. https://doi.org/10.1111/cas.12274
Yamasaki M, Yasuda T, Yano M et al (2017) Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol 28:116–120. https://doi.org/10.1093/annonc/mdw439
Hagi T, Makino T, Yamasaki M et al (2019) Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol 26:4754–4764. https://doi.org/10.1245/s10434-019-07744-7
Takahashi H, Arimura Y, Yamashita K et al (2010) Phase I/II study of docetaxel/cisplatin/fluorouracil combination chemotherapy against metastatic esophageal squamous cell carcinoma. J Thorac Oncol 5:122–128. https://doi.org/10.1097/JTO.0b013e3181c1ffd5
Tamura S, Imano M, Takiuchi H et al (2012) Phase II study of docetaxel, cisplatin and 5-fluorouracil (DCF) for metastatic esophageal cancer (OGSG 0403). Anticancer Res 32:1403–1408
Yamagata Y, Saito K, Hirano K, Oya M (2020) Long-term outcomes and safety of radical transmediastinal esophagectomy with preoperative docetaxel, cisplatin, and 5-fluorouracil combination chemotherapy for locally advanced squamous cell carcinoma of the thoracic esophagus. World J Surg Oncol 18:252. https://doi.org/10.1186/s12957-020-02023-2
Yamasaki M, Miyata H, Tanaka K et al (2011) Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology 80:307–313. https://doi.org/10.1159/000329806
Yoshida Y, Komori K, Aoki M, Sandou M, Takagi M, Uejima E (2018) Efficacy of pegfilgrastim administration in patients with esophageal cancer treated with docetaxel, cisplatin, and 5-fluorouracil. Pharmazie 73:613–616. https://doi.org/10.1691/ph.2018.8576
Ishikawa T, Yasuda T, Okayama T et al (2019) Early administration of pegfilgrastim for esophageal cancer treated with docetaxel, cisplatin, and fluorouracil: a phase II study. Cancer Sci 110:3754–3760. https://doi.org/10.1111/cas.14218
Ohkura Y, Ueno M, Udagawa H (2019) Risk factors for febrile neutropenia and effectiveness of primary prophylaxis with pegfilgrastim in patients with esophageal cancer treated with docetaxel, cisplatin, and 5-fluorouracil. World J Surg Oncol 17:125. https://doi.org/10.1186/s12957-019-1665-x
Bosly A, Bron D, Van Hoof A et al (2008) Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol 87:277–283. https://doi.org/10.1007/s00277-007-0399-y
Crawford J, Denduluri N, Patt D et al (2020) Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer. Support Care Cancer 28:925–932. https://doi.org/10.1007/s00520-019-04875-1
Hanna RK, Poniewierski MS, Laskey RA et al (2013) Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol 129:74–80. https://doi.org/10.1016/j.ygyno.2012.12.017
Hirakawa T, Yamaguchi H, Yokose N, Gomi S, Inokuchi K, Dan K (2010) Importance of maintaining the relative dose intensity of CHOP-like regimens combined with rituximab in patients with diffuse large B-cell lymphoma. Ann Hematol 89:897–904. https://doi.org/10.1007/s00277-010-0956-7
Nomura H, Hatogai K, Maki Y et al (2020) Risk factors for febrile neutropenia in neoadjuvant docetaxel, cisplatin, and 5-fluorouracil chemotherapy for esophageal cancer. Support Care Cancer 28:1849–1854. https://doi.org/10.1007/s00520-019-05001-x
Chen LK, Woo J, Assantachai P et al (2020) Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21:300–307. https://doi.org/10.1016/j.jamda.2019.12.012
Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM (2018) Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle 9:315–325. https://doi.org/10.1002/jcsm.12267
Kurk S, Peeters P, Stellato R et al (2019) Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle 10:803–813. https://doi.org/10.1002/jcsm.12436
Shachar SS, Deal AM, Weinberg M et al (2017) Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res 23:658–665. https://doi.org/10.1158/1078-0432.CCR-16-0940
Mariette C, De Botton ML, Piessen G (2012) Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol 19:2128–2134. https://doi.org/10.1245/s10434-012-2225-6
Kamitani N, Migita K, Matsumoto S et al (2019) Association of skeletal muscle loss with the long-term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg Today 49:1022–1028. https://doi.org/10.1007/s00595-019-01846-1
Miyata H, Sugimura K, Motoori M, et al (2017) Clinical assessment of sarcopenia and changes in body composition during neoadjuvant chemotherapy for esophageal cancer. Anticancer Res 37:3053–3059. https://doi.org/10.21873/anticanres.11660
Chien MY, Huang TY, Wu YT (2008) Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc 56:1710–1715. https://doi.org/10.1111/j.1532-5415.2008.01854.x
Hamaguchi Y, Kaido T, Okumura S et al (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 32:1200–1205. https://doi.org/10.1016/j.nut.2016.04.003
Janssen I, Heymsfield SB, Baumgartner RN (1985) Ross R (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89:465–471. https://doi.org/10.1152/jappl.2000.89.2.465
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. https://doi.org/10.1159/000180580
Saito Y, Kobayashi M, Yamada T et al (2017) Premedication with intravenous magnesium has a protective effect against cisplatin-induced nephrotoxicity. Support Care Cancer 25:481–487. https://doi.org/10.1007/s00520-016-3426-5
Smith TJ, Khatcheressian J, Lyman GH et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205. https://doi.org/10.1200/JCO.2006.06.4451
Aogi K, Takeuchi H, Saeki T et al (2021) Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology clinical practice guidelines for antiemesis. Int J Clin Oncol 26:1–17. https://doi.org/10.1007/s10147-020-01818-3
Masaoka T (2004) Evidence-based recommendations for antimicrobial use in febrile neutropenia in Japan: executive summary. Clin Infect Dis 39(Suppl 1):S49-52. https://doi.org/10.1086/383054
Lyman GH, Delgado DJ (2003) Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 98:2402–2409. https://doi.org/10.1002/cncr.11827
Aagaard T, Roen A, Reekie J, et al (2018) Development and validation of a risk score for febrile neutropenia after chemotherapy in patients with cancer: the FENCE score. JNCI Cancer Spectr 2:pky053. https://doi.org/10.1093/jncics/pky053
Gusella M, Toso S, Ferrazzi E, Ferrari M, Padrini R (2002) Relationships between body composition parameters and fluorouracil pharmacokinetics. Br J Clin Pharmacol 54:131–139. https://doi.org/10.1046/j.1365-2125.2002.01598.x
Halvorsen TO, Valan CD, Slaaen M, Gronberg BH (2020) Associations between muscle measures, survival, and toxicity in patients with limited stage small cell lung cancer. J Cachexia Sarcopenia Muscle 11:1283–1290. https://doi.org/10.1002/jcsm.12583
Prado CM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13:3264–3268. https://doi.org/10.1158/1078-0432.CCR-06-3067
Williams GR, Deal AM, Shachar SS et al (2018) The impact of skeletal muscle on the pharmacokinetics and toxicity of 5-fluorouracil in colorectal cancer. Cancer Chemother Pharmacol 81:413–417. https://doi.org/10.1007/s00280-017-3487-2
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B (2005) Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065. https://doi.org/10.2165/00003088-200544100-00004
Funding
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of the study. Katsuhiko Nara and Takehito Yamamoto performed the data collection and analysis. The first draft of the manuscript was written by Katsuhiko Nara, Takehito Yamamoto, Yasuyoshi Sato, Koichi Yagi, Koichiro Kawasaki, Tetsuro Toriumi, and Tappei Takada, and all the authors commented on the previous versions of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of this retrospective, observational study was approved by the Institutional Review Board of the Graduate School of Medicine and Faculty of Medicine, University of Tokyo (Approval number: 2529). The institutional review board granted an opt-out recruitment approach and waived the need for written informed consent from each patient. This study was conducted in accordance with the Declaration of Helsinki and its latest amendment.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nara, K., Yamamoto, T., Sato, Y. et al. Low pretherapy skeletal muscle mass index is associated with an increased risk of febrile neutropenia in patients with esophageal cancer receiving docetaxel + cisplatin + 5-fluorouracil (DCF) therapy. Support Care Cancer 31, 150 (2023). https://doi.org/10.1007/s00520-023-07609-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07609-6