Abstract
Purpose
Mounting evidence suggests that the gut microbiome influences radiotherapy efficacy and toxicity by modulating immune signalling. However, its contribution to radiotherapy outcomes in head and neck cancer (HNC) is yet to be investigated. This study, therefore, aimed to uncover associations between an individual’s pre-therapy gut microbiota and (i) severity of radiotherapy-induced oral mucositis (OM), and (ii) recurrence risk in patients with HNC.
Methods
In this prospective pilot study, 20 patients with HNC scheduled to receive radiotherapy or chemoradiotherapy were recruited. Stool samples were collected before treatment and microbial composition was analysed using 16S rRNA gene sequencing. OM severity was assessed using the NCI-CTCAE scoring system. Patients were also followed for 12 months of treatment completion to assess tumour recurrence.
Results
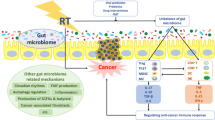
Overall, 80% of the patients were male with a median age of 65.5 years. Fifty-three percent experienced mild/moderate OM while 47% developed severe OM. Furthermore, 18% experienced tumour relapse within 1 year of treatment completion. A pre-treatment microbiota enriched of Eubacterium, Victivallis, and Ruminococcus was associated with severe OM. Conversely, a higher relative abundance of immunomodulatory microbes Faecalibacterium, Prevotella, and Phascolarctobacterium was associated with a lower risk of tumour recurrence.
Conclusion
Our results indicate that a patient’s gut microbiota composition at the start of treatment is linked to OM severity and recurrence risk. We now seek to validate these findings to determine their ability to predict treatment outcomes in HNC, with the goal of using this data to inform second-generation microbial therapeutics to optimise treatment outcomes for patients with HNC.





Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Yeh SA (2010) Radiotherapy for head and neck cancer. Semin Plast Surg 24(2):127–136. https://doi.org/10.1055/s-0030-1255330
Gooi Z, Fakhry C, Goldenberg D, Richmon J, Kiess AP (2016) AHNS Series: do you know your guidelines? Principles of radiation therapy for head and neck cancer: a review of the National Comprehensive Cancer Network guidelines. Head Neck 38(7):987–992. https://doi.org/10.1002/hed.24448
Maria OM, Eliopoulos N, Muanza T (2017) Radiation-induced oral mucositis. Front Oncol 7:89. https://doi.org/10.3389/fonc.2017.00089
Bachour PC, Sonis ST (2018) Predicting mucositis risk associated with cytotoxic cancer treatment regimens: rationale, complexity, and challenges. Curr Opin Support Palliat Care 12(2):198–210. https://doi.org/10.1097/spc.0000000000000339
Wardill HR, Sonis ST, Blijlevens NMA, Van Sebille YZA, Ciorba MA, Loeffen EAH, Cheng KKF, Bossi P, Porcello L, Castillo DA, Elad S, Bowen JM, On behalf of The Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral O (2020) Prediction of mucositis risk secondary to cancer therapy: a systematic review of current evidence and call to action. Supp Care Cancer 28(11):5059–5073. https://doi.org/10.1007/s00520-020-05579-7
Begg AC, Stewart FA, Vens C (2011) Strategies to improve radiotherapy with targeted drugs. Nature Reviews Cancer 11(4):239–253
Orth M, Lauber K, Niyazi M, Friedl AA, Li M, Maihöfer C, Schüttrumpf L, Ernst A, Niemöller OM, Belka C (2014) Current concepts in clinical radiation oncology. Radiation and environmental biophysics 53(1):1–29. https://doi.org/10.1007/s00411-013-0497-2
Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM (2021) Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. https://doi.org/10.1038/s41577-021-00568-1
Roy S, Trinchieri G (2017) Microbiota: a key orchestrator of cancer therapy. Nature Reviews Cancer 17:271–285
Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity. Nature Reviews Gastroenterology and Hepatology 14(6):356–365
Al-Qadami G, Van Sebille Y, Le H, Bowen J (2019) Gut microbiota: implications for radiotherapy response and radiotherapy-induced mucositis. Expert Review of Gastroenterology & Hepatology 13:485–496. https://doi.org/10.1080/17474124.2019.1595586
Tonneau M, Elkrief A, Pasquier D, Socorro TPD, Chamaillard M, Bahig H, Routy B (2021) The role of the gut microbiome on radiation therapy efficacy and gastrointestinal complications: a systematic review. Radiotherapy and Oncology 156:1–9. https://doi.org/10.1016/j.radonc.2020.10.033
Covington JA, Wedlake L, Andreyev J, Ouaret N, Thomas MG, Nwokolo CU, Bardhan KD, Arasaradnam RP (2012) The detection of patients at risk of gastrointestinal toxicity during pelvic radiotherapy by electronic nose and FAIMS: a pilot study. Sensors (Basel) 12(10):13002–13018. https://doi.org/10.3390/s121013002
Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gómez L, Verginadis I, Bittinger K, Pustylnikov S, Pierini S, Perales-Linares R, Blair IA, Mesaros CA, Snyder NW, Bushman F, Koumenis C, Facciabene A (2020) Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest 130(1):466–479. https://doi.org/10.1172/jci124332
Yang K, Hou Y, Zhang Y, Liang H, Sharma A, Zheng W, Wang L, Torres R, Tatebe K, Chmura SJ, Pitroda SP, Gilbert JA, Fu Y-X, Weichselbaum RR (2021) Suppression of local type I interferon by gut microbiota–derived butyrate impairs antitumor effects of ionizing radiation. J Exp Med 218(3):e20201915. https://doi.org/10.1084/jem.20201915
Nenclares P, Bhide SA, Sandoval-Insausti H, Pialat P, Gunn L, Melcher A, Newbold K, Nutting CM, Harrington KJ (2020) Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur J Cancer 131:9–15. https://doi.org/10.1016/j.ejca.2020.02.047
Shu Z, Li P, Yu B, Huang S, Chen Y (2020) The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis: a systematic review and meta-analysis. Oral Oncol 102:104559. https://doi.org/10.1016/j.oraloncology.2019.104559
Trotti A, Colevas AD, Setser A, Basch E (2007) Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol 25(32):5121–5127. https://doi.org/10.1200/jco.2007.12.4784
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60. https://doi.org/10.1186/gb-2011-12-6-r60
Wade WG (2006) The genus Eubacterium and related genera. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer US, New York, NY, pp 823–835. https://doi.org/10.1007/0-387-30744-3_28
De Maesschalck C, Van Immerseel F, Eeckhaut V, De Baere S, Cnockaert M, Croubels S, Haesebrouck F, Ducatelle R, Vandamme P (2014) Faecalicoccus acidiformans gen. nov., sp. nov., isolated from the chicken caecum, and reclassification of Streptococcus pleomorphus (Barnes et al. 1977), Eubacterium biforme (Eggerth 1935) and Eubacterium cylindroides (Cato et al. 1974) as Faecalicoccus pleomorphus comb. nov., Holdemanella biformis gen. nov., comb. nov. and Faecalitalea cylindroides gen. nov., comb. nov., respectively, within the family Erysipelotrichaceae. Int J Syst Evol Microbiol 64(Pt_11):3877–3884. https://doi.org/10.1099/ijs.0.064626-0
Pujo J, Petitfils C, Le Faouder P, Eeckhaut V, Payros G, Maurel S, Perez-Berezo T, Van Hul M, Barreau F, Blanpied C, Chavanas S, Van Immerseel F, Bertrand-Michel J, Oswald E, Knauf C, Dietrich G, Cani PD, Cenac N (2020) Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 70:1088–1097. https://doi.org/10.1136/gutjnl-2020-321173
Schippa S, Iebba V, Santangelo F, Gagliardi A, De Biase RV, Stamato A, Bertasi S, Lucarelli M, Conte MP, Quattrucci S (2013) Cystic fibrosis transmembrane conductance regulator (CFTR) allelic variants relate to shifts in faecal microbiota of cystic fibrosis patients. PLoS One 8(4):e61176. https://doi.org/10.1371/journal.pone.0061176
Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A (2018) Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European Gastroenterol J 6(10):1496–1507. https://doi.org/10.1177/2050640618804444
Maity C, Gupta AK, Saroj DB, Biyani A, Bagkar P, Kulkarni J, Dixit Y (2020) Impact of a gastrointestinal stable probiotic supplement Bacillus coagulans LBSC on human gut microbiome modulation. Journal of Dietary Supplements 18:577–596. https://doi.org/10.1080/19390211.2020.1814931
Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE (2013) Alterations in the gut microbiota associated with HIV-1 infection. Cell host & microbe 14(3):329–339. https://doi.org/10.1016/j.chom.2013.08.006
Plugge CM, Zoetendal EG (2014) The family Victivallaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes: other major lineages of bacteria and the archaea. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 1019–1021. https://doi.org/10.1007/978-3-642-38954-2_150
Sánchez-Alcoholado L, Ordóñez R, Otero A, Plaza-Andrade I, Laborda-Illanes A, Medina JA, Ramos-Molina B, Gómez-Millán J, Queipo-Ortuño MI (2020) Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal cancer. Int J Mol Sci 21(18):6782. https://doi.org/10.3390/ijms21186782
Cornejo-Pareja I, Ruiz-Limón P, Gómez-Pérez AM, Molina-Vega M, Moreno-Indias I, Tinahones FJ (2020) Differential microbial pattern description in subjects with autoimmune-based thyroid diseases: a pilot study. Journal of personalized medicine 10(4):192. https://doi.org/10.3390/jpm10040192
Li N, Wang X, Sun C, Wu X, Lu M, Si Y, Ye X, Wang T, Yu X, Zhao X, Wei N, Wang X (2019) Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol 19(1):191–191. https://doi.org/10.1186/s12866-019-1552-1
La Reau AJ, Meier-Kolthoff JP, Suen G (2016) Sequence-based analysis of the genus Ruminococcus resolves its phylogeny and reveals strong host association. Microbial genomics 2(12):e000099. https://doi.org/10.1099/mgen.0.000099
Togo AH, Diop A, Bittar F, Maraninchi M, Valero R, Armstrong N, Dubourg G, Labas N, Richez M, Delerce J, Levasseur A, Fournier P-E, Raoult D, Million M (2018) Description of Mediterraneibacter massiliensis, gen. nov., sp. nov., a new genus isolated from the gut microbiota of an obese patient and reclassification of Ruminococcus faecis, Ruminococcus lactaris, Ruminococcus torques, Ruminococcus gnavus and Clostridium glycyrrhizinilyticum as Mediterraneibacter faecis comb. nov., Mediterraneibacter lactaris comb. nov., Mediterraneibacter torques comb. nov., Mediterraneibacter gnavus comb. nov. and Mediterraneibacter glycyrrhizinilyticus comb. nov. Antonie Van Leeuwenhoek 111(11):2107–2128. https://doi.org/10.1007/s10482-018-1104-y
Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH (2010) Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105(11):2420–2428. https://doi.org/10.1038/ajg.2010.281
Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J (2019) Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A 116(26):12672–12677. https://doi.org/10.1073/pnas.1904099116
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS et al (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359(6371):97–103. https://doi.org/10.1126/science.aan4236
Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, Zheng H, Yao C, Wang Y, Lu S (2019) The diversity of gut microbiome is associated with favorable responses to anti–programmed death 1 immunotherapy in Chinese patients with NSCLC. Journal of Thoracic Oncology 14(8):1378–1389. https://doi.org/10.1016/j.jtho.2019.04.007
Limeta A, Ji B, Levin M, Gatto F, Nielsen J (2020) Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI insight 5(23):e140940. https://doi.org/10.1172/jci.insight.140940
Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, Zhang X, Gong J-f, Li J, Lu M, Wang X, Zhou J, Lu Z, Zhang Q, Tzeng DTW, Bi D, Tan Y, Shen L (2020) The gut microbiome is associated with clinical response to anti–PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunology Research 8(10):1251–1261. https://doi.org/10.1158/2326-6066.cir-19-1014
Al-Qadami G, Van Sebille Y, Bowen J, Wardill H (2022) Oral-gut microbiome axis in the pathogenesis of cancer treatment-induced oral mucositis. Front Oral Health 3:881949. https://doi.org/10.3389/froh.2022.881949
Liu J, Liu C, Yue J (2021) Radiotherapy and the gut microbiome: facts and fiction. Radiat Oncol 16(1):9. https://doi.org/10.1186/s13014-020-01735-9
Reis Ferreira M, Pasto A, Ng T, Patel V, Guerrero Urbano T, Sears C, Wade WG (2022) The microbiota and radiotherapy for head and neck cancer: what should clinical oncologists know? Cancer Treat Rev 109:102442. https://doi.org/10.1016/j.ctrv.2022.102442
Olsen I, Yamazaki K (2019) Can oral bacteria affect the microbiome of the gut? J Oral Microbiol 11(1):1586422. https://doi.org/10.1080/20002297.2019.1586422
Wardill HR, Chan RJ, Chan A, Keefe D, Costello SP, Hart NH (2022) Dual contribution of the gut microbiome to immunotherapy efficacy and toxicity: supportive care implications and recommendations. Supportive Care in Cancer 30(8):6369–6373. https://doi.org/10.1007/s00520-022-06948-0
Acknowledgements
We would like to sincerely thank the nurses and radiation oncologists at the Department of Radiation Oncology (Royal Adelaide Hospital) for their assistance during the recruitment process.
Funding
This study was supported by the Royal Adelaide Hospital Clinical Project Grant (CI Wardill).
Author information
Authors and Affiliations
Contributions
GA, JB, YVS, KS, and HL made substantial contributions to study conceptualisation and design (GA, JB, and HL); patient recruitment and sample collection (GA and HL); DNA extraction and microbial data analysis (GA and KS); and drafting (GA) and revising the manuscript (GA, JB, YVS, KS, and HL). HW is PI for the study, securing relevant ethical approvals and seed funding as well as contributing to data analysis and revising the manuscript. MD and JV contributed to the data analysis and interpretation and reviewing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Royal Adelaide Hospital Human Research Ethics Committee (HREC/17/RAH/533 (R20171131)).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hannah Wardill and Hien Le have shared senior authorship.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Qadami, G., Bowen, J., Van Sebille, Y. et al. Baseline gut microbiota composition is associated with oral mucositis and tumour recurrence in patients with head and neck cancer: a pilot study. Support Care Cancer 31, 98 (2023). https://doi.org/10.1007/s00520-022-07559-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-022-07559-5