Abstract
Purpose
The status and associated factors of the health-related quality of life of non-small-cell lung cancer (NSCLC) patients under targeted anti-cancer therapy have not been investigated. Self-management and coping style have been proven to be closely related to patients’ health-related quality of life. Based on these observations, this study was designed to firstly assess the status of health-related quality of life, and then explore the relationships among coping styles, self-management, and health-related quality of life of NSCLC patients with skin adverse drug reactions under targeted therapy.
Methods
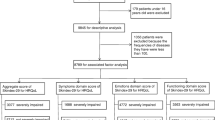
We performed a cross-sectional study including 536 NSCLC patients with skin adverse drug reactions under targeted therapy in cancer clinics of three hospitals in China between May 2020 and May 2021. Structured questionnaires, including the Functional Assessment of Cancer Therapy-Epidermal Growth Factor Receptor Inhibitor 18, Cancer Patient Self-management Evaluation Scale, and Medical Coping Modes Questionnaire, were used to collect data. Relationships among coping style, self-management, and health-related quality of life were identified by Pearson correlation analysis and a multiple linear regression algorithm.
Results
The total score of health-related quality of life was 46 ± 12.84 in 536 NSCLC patients with skin adverse drug reactions undergoing targeted therapy. Health-related quality of life was positively correlated with self-management (r = 0.785, P < 0.01) and facing (r = 0.807, P < 0.01) and negatively correlated with yield (r = − 0.718, P < 0.01), avoidance (r = − 0.711, P < 0.01), and the severity of skin adverse reactions (r = − 0.722, P = 0.000). Via multiple linear regression analysis, we identified some significant factors associated with health-related quality of life, including age, education level, combination of medicine, Charlson Comorbidity Index, stages of disease, facing, yield, symptom management, daily activity management, psychological and emotional management, self-efficacy, and self-management (P < 0.05).
Conclusions
NSCLC patients with skin adverse drug reactions undergoing targeted therapy generally had a compromised health-related quality of life. The critical factors that were associated with the status of health-related quality of life were age, education level, comorbidity, the combinatorial application of drugs, stage of disease, self-management, and coping styles.

Similar content being viewed by others
Data availability
The data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author who was an organizer of the study.
Code availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) CA Cancer J Clin 71(3):209–249
Rongshou Z, Siwei Z, Hongmei Z, Changfa X (2022) J National Cancer Cent 12(3):525–537
Galvez-Nino M, Ruiz R, Pinto JA, Roque K, Mas L (2020) Lung Cancer 198(1):195–200
Thomas A, Chen Y, Yu T, Jakopovic M (2015) Front Oncol 5(33):113–119
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Int J Cancer 136(5):E359–E386
Liu X, Luo X, Jiang C, Zhao H (2019) Clin Genet 95(6):569–574
Wilkes GM (2018) Asia Pac J Oncol Nurs 5(2):137–155
Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, Gossai A, Frampton GM, Torres AZ, Lehnert EM (2019) JAMA J Am Med Assoc 321(14):1391–1402
Staats H, Cassidy C, Kelso J, Mack S, Nemunaitis J (2020) Biomedicine 5(13):27–49
Wu Y, Zhou C, Lu S, Qin S (2019) Lung Cancer 130(20):18–24
Ettinger DS, Wood DE, Akerley W, Bazhenova LA (2016) J Natl Compr Canc Netw Jnccn 14(3):255–264
Wang MC, Wang CL, Chen TL, Chang WC, Lu JJ, Chang PY, Chiou CC (2017) Clin Chem Lab Med 55(12):1979–1986
Kale HP, Mays AP, Nadpara PA, Slattum PW (2019) Urol Oncol 356(37):19–28
Peng Y, Li Q, Zhang J, Shen W, Zhang X, Sun C, Cui H (2018) Biosci Trends 33(32):3817–3825
JLee JL, Jeong Y (2019) Cancer Nurs 42(6):475–483
Barrios DM, Phillips GS, Freites-Martinez A, Hsu M, Lacouture ME (2019) J Eur Acad Dermatol 34(6):27–39
Chan JC, Lee YH, Liu CY, Shih HH, Tang WR (2019) J Nurs Res 27(6):51–59
Yagasaki K, Komatsu H, Soejima K, Naoki K, Hamamoto Y (2018) Asia Pac J Oncol Nurs 5(2):172–177
Sano K, Nakadate K, Hanada K (2020) BMC Cancer 20(1):279–287
Komatsu H, Yagasaki K, Yamaguchi T, Mori A, Tamura K (2020) Eur J Oncol Nurs 23(8):1017–1028
Wagner LI, Lacouture ME (2007) Oncology 21(5):34–36
Wagner LI, Berg SR, Gandhi M, Hlubocky FJ (2013) Support Care Cancer 21(4):1033–1041
Liao Y-C, Liao W-Y, Sun J-L, Ko J-C, Yu C-J (2018) Support Care Cancer 26(2):989–996
Yuan M, Huang LL, Chen JH, Wu J, Xu Q (2019) Signal Transduct Target Ther 55(5):103–111
Tantoy IY, Cataldo JK, Aouizerat BE, Dhruva A (2016) Cancer Nurs 39(6):437–445
Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E (2018) Clin Colorectal Canc 17(2):85–96
Huang FF, Yang Q, Zhang J, Han XY, Zhang JP, Chirico A (2018) PLoS ONE 13(9):332–339
Chirico A, Lucidi F, Merluzzi T, Alivernini F, Giordano A (2017) Oncotarget 8(22):36800–36811
Chen H, Senan S, Nossent EJ, Boldt RG, Warner A, Palma DA, Louie AV (2017) Int J Radiat Oncol 47(6):327–336
Charan J, Biswas T (2013) Indian J Psychol Med 35(2):121–126
Qing L, Yanmei P, Jingyi Z, Shen W (2020) J China-Japan Friendship Hospital 34(5):263–267
Lingling C (2017) S Yuqian. Chin J Nurs 52(9):1082–1087
Sun V, Reb A, Debay M, Fakih M, Ferrell B (2021) J Cancer Educ 36(4):421–428
Xiaohong S, Qianjin J (2000) Chin J Behav Med Brain Sci 9(1):18–20
Wu XD, Qin HY, Zhang JE, Zheng MC, Xin MZ, Liu L, Wu XJ, Jiang CN, Zhang MF (2015) Eur J Oncol Nurs 21(5):725–732
He Y, Jian H, Yan M, Zhu J, Chen J (2019) BMJ Open 9(5):233–239
Hauke J, Kossowski T (2011) Quaest Geogr 30(2):87–93
Barnes TA, Amir E, Templeton AJ, Gomez-Garcia S, Navarro B, Seruga B, Ocana A (2017) Cancer Treat Rev 56(6):1–7
Yamamoto K, Yano I (2018) Med Oncol 35(2):16–26
Du R, Wang X, Ma L, Larcher LM, Wang T (2021) BMC Cancer 21(1):231–239
Occhipinti M, Brambilla M, Galli G, Manglaviti S, Prelaj A, Ferrara R, Toma AD, Beninato T, Zattarin E, Proto C (2021) J Thorac Oncol 16(4):1257–1265
Chen CB, Wu MY, Yee NC, Lu CW, Wu J, Pei-Han K, Yang CK, Peng MT, Huang CY, Chang WC (2018) Cancer Manag Res 10:1259–1273
Deutsch A, Leboeuf NR, Lacouture ME, Mclellan BN (2020) Proc Am Soc Clin Oncol 40(40):485–500
He Y, Jian H, Yan M, Zhu J, Chen J (2019) BMJ Open 9(5):236–242
Cheng T, Respiration DO (2016) China Health Industry 13(16):532–536
De W, Derijcke S, Galdermans D, Daenen M, Surmont V, Droogh ED, Lefebure A, Saenen E, Vandenbroucke E, Morel AM (2020) Clin Lung Cancer 2(22):146–152
Kafatos G, Dube S, Burdon P, Demonty G, Flinois A, Leclerc M, Lowe K, Feudjo-Tepie M, Segaert S (2020) Clin Colorectal Canc 19(2):100–108
Sun V, Reb A, Debay M, Fakih M, Ferrell B (2021) J Cancer Educ 25(4):788–796
Chen Q, Di S, Ying Y, Yue Z, Xiaoying Z (2019) Qual Life Res Int J Qual Life Asp Treat Care Rehabil 67(5):1245–1253
Liao Y-C, Liao W-Y, Sun J-L, Ko J-C, Yu C-J (2018) Support Care Cancer 12(23):236–243
Huang W, Li J, Qiu F, Wu X, Zhu S (2020) J Clin Pharm Ther 45(12):29–39
Acknowledgements
We thank the First Affiliated Hospital of Zhengzhou University and Henan Tumor Hospital and the First Affiliated Hospital of Henan University of Science and Technology for their assistance and support and the Follow-up Registration System and data provided by the Follow-up Center of the First Affiliated Hospital of Zhengzhou University. We also thank the oncologists and nurses of the three hospitals. We appreciate all participants for their generous participation.
Funding
This study received financial support from the China Postdoctoral Science Foundation in 2018 (2018M630839) and the National Natural Science Foundation of China (no. 81773175)
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. WT and CCY were responsible for the overall design and quality control of the study as well as the communication with the hospital and departments reviewed. DRF, WX, and WT made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; WX, LML, and MLX were involved in drafting the manuscript or revising it critically for important intellectual content; ZHY contributed to the training and management of nurses and students who collected data. DRF, ZHY, WX, LML, CCY, and WT gave final approval of the version to be published. MLX contributed to data analysis and solving statistical problems. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content; DRF, CCY, and WT agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of our university and administrative permissions were obtained from directors of oncology departments. All methods were carried out in accordance with relevant guidelines and regulations. Information about the study was provided to the participants, and we obtained written informed consent from all participants prior to their inclusion in the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, R., Wang, X., Zhou, H. et al. The health-related quality of life of lung cancer patients with EGFR-TKI-related skin adverse drug reactions and its relationship with coping style and self-management. Support Care Cancer 30, 9889–9899 (2022). https://doi.org/10.1007/s00520-022-07451-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07451-2