Abstract
Purpose
Diversion of tryptophan to tumoral hormonal production has been suggested to result in psychiatric illnesses in neuroendocrine tumors (NET). We measured the occurrence of psychiatric illness after NET diagnosis and compare it to colon cancer (CC).
Methods
We conducted a population-based retrospective cohort study. Adults with NET were matched 1:1 to CC (2000–2019). Psychiatric illness was defined by mental health diagnoses and mental health care use after a cancer diagnosis, categorized as severe, other, and none. Cumulative incidence functions accounted for death as a competing risk.
Results
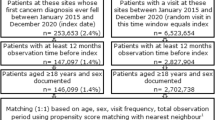
A total of 11,223 NETs were matched to CC controls. Five-year cumulative incidences of severe psychiatric illness for NETs vs. CC was 7.7% (95%CI 7.2–8.2%) vs 7.6% (95%CI 7.2–8.2%) (p = 0.50), and that of other psychiatric illness was 32.9% (95%CI 32.0–33.9%) vs 31.6% (95%CI 30.8–32.6%) (p = 0.005). In small bowel and lung NETs, 5-year cumulative incidences of severe (8.1% [95%CI 7.3–8.9%] vs. 7.0% [95%CI 6.3–7.8%]; p = 0.01) and other psychiatric illness (34.7% [95%CI 33.3–36.1%] vs. 31.1% [95%CI 29.7–32.5%]; p < 0.01) were higher than for matched CC. The same was observed for serotonin-producing NETs for both severe (7.9% [95%CI 6.5–9.4%] vs. 6.8% [95%CI 5.5–8.2%]; p = 0.02) and other psychiatric illness (35.4% [95%CI 32.8–38.1%] vs. 31.9% [95%CI 29.3–34.4%]; p = 0.02).
Conclusions
In all NETs, there was no difference observed in the incidence of psychiatric illness compared to CC. For sub-groups of small bowel and lung NETs and of serotonin-producing NETs, the incidence of psychiatric illness was higher than for CC. These data suggest a signal towards a relationship between those sub-groups of NETs and psychiatric illness.





Similar content being viewed by others
References
Modlin IM, Oberg K, Chung DC et al (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9(61):72. https://doi.org/10.1016/s1470-2045(07)70410-2
Zikusoka MN, Kidd M, Eick G et al (2005) The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer 104:2292–2309. https://doi.org/10.1002/cncr.21451
Hallet J, Cukier M, Saskin R, Liu N (2015) Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 121(589):597. https://doi.org/10.1002/cncr.29099
Dasari A, Shen C, Halperin D et al (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3(1335):8. https://doi.org/10.1001/jamaoncol.2017.0589
Russo S, Nielen MMA, Boon JC et al (2003) Neuropsychological investigation into the carcinoid syndrome. Psychopharmacology 168:324–328. https://doi.org/10.1007/s00213-003-1455-5
Shah GM, Shah RG, Veillette H et al (2005) Biochemical assessment of niacin deficiency among carcinoid cancer patients. Am J Gastroenterol 100:2307–2314. https://doi.org/10.1111/j.1572-0241.2005.00268.x
Pasieka JL, Longman RS, Chambers AJ et al (2014) Cognitive impairment associated with carcinoid syndrome. Ann Surg 259(355):359. https://doi.org/10.1097/sla.0b013e318288ff6d
Wefel JS, Saleeba AK, Buzdar AU, Meyers CA (2010) Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 116:3348–3356. https://doi.org/10.1002/cncr.25098
Cimprich B, Reuter-Lorenz P, Nelson J et al (2010) Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol 32:324–331. https://doi.org/10.1080/13803390903032537
Miller AH, Ancoli-Israel S, Bower JE et al (2008) Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 26(971):982. https://doi.org/10.1200/jco.2007.10.7805
Scandurra C, Modica R, Maldonato NM et al (2021) Quality of life in patients with neuroendocrine neoplasms: the role of severity, clinical heterogeneity, and resilience. J Clin Endocrinol Metab 106:e316–e327. https://doi.org/10.1210/clinem/dgaa760
Shah RK, Arjmand E, Roberson DW et al (2011) Variation in surgical time-out and site marking within pediatric otolaryngology. Archives Otolaryngol–Head Neck Surg 137:69–73. https://doi.org/10.1001/archoto.2010.232
Lehmann J (1982) Tryptophan deficiency stupor–a new psychiatric syndrome. Acta Psychiatr Scand Suppl 300(1):57
Hanna SM (1965) Carcinoid syndrome associated with psychosis. Postgrad Med J 41:566–567. https://doi.org/10.1136/pgmj.41.479.566
Trivedi S (1984) Psychiatric symptoms in carcinoid syndrome. J Indian Med Assoc 82:292–294
Major LF, Brown GL, Wilson WP (1973) Carcinoid and psychiatric symptoms. South Med J 66(787):790
Benchimol EI, Smeeth L, Guttmann A et al (2015) The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 12(e1001885):22. https://doi.org/10.1371/journal.pmed.1001885
Canada H (2004) Canada Health Act. https://www.canada.ca/en/health-canada/services/health-care-system/canada-health-care-system-medicare/canada-health-act.html. Accessed 29 Feb 2020
Hallet J, Law CHL, Karanicolas PJ et al (2015) Rural-urban disparities in incidence and outcomes of neuroendocrine tumors: a population-based analysis of 6271 cases. Cancer 121:2214–2221. https://doi.org/10.1002/cncr.29338
Hallet J, Law CHL, Cheung M et al (2017) Patterns and drivers of costs for neuroendocrine tumor care: a comparative population-based analysis. Ann Surg Oncol 24:3312–3323. https://doi.org/10.1245/s10434-017-5986-0
Robles SC, Marrett LD, Clarke EA, Risch HA (1988) An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 41(495):501
Clarke EA, Marrett LD, Kreiger N (1991) Cancer registration in Ontario: a computer approach. IARC Sci Publ. 246 – 257.
Juurlink D, Preyra C, Croxford R Canadian institute for health information discharge abstract database: a validation study
Vigod SN, Dennis CL, Kurdyak PA et al (2014) Fertility rate trends among adolescent girls with major mental illness: a population-based study. Pediatrics 133:e585-591. https://doi.org/10.1542/peds.2013-1761
Kurdyak P, Lin E, Green D, Vigod S (2015) Validation of a population-based algorithm to detect chronic psychotic illness. Can J Psychiatry 60:362–368. https://doi.org/10.1177/070674371506000805
Mahar AL, Kurdyak P, Hanna TP et al (2020) The effect of a severe psychiatric illness on colorectal cancer treatment and survival: a population-based retrospective cohort study. PLoS One 15:e0235409. https://doi.org/10.1371/journal.pone.0235409
Mahar AL, Kurdyak P, Hanna TP et al (2020) Cancer staging in individuals with a severe psychiatric illness: a cross-sectional study using population-based cancer registry data. BMC Cancer 20:476. https://doi.org/10.1186/s12885-020-06943-w
Ruggeri M, Leese M, Thornicroft G et al (2000) Definition and prevalence of severe and persistent mental illness. Br J Psychiatry 177:149–155. https://doi.org/10.1192/bjp.177.2.149
Heggestad T, Lilleeng SE, Ruud T (2011) Patterns of mental health care utilisation: distribution of services and its predictability from routine data. Soc Psychiatry Psychiatr Epidemiol 46:1275–1282. https://doi.org/10.1007/s00127-010-0295-y
Kisely S, Forsyth S, Lawrence D (2016) Why do psychiatric patients have higher cancer mortality rates when cancer incidence is the same or lower? Aust N Z J Psychiatry 50:254–263. https://doi.org/10.1177/0004867415577979
Lora A, Bezzi R, Erlicher A (2007) Estimating the prevalence of severe mental illness in mental health services in Lombardy (Italy). Community Ment Health J 43:341–357. https://doi.org/10.1007/s10597-006-9078-z
Kralj B (2000) Measuring “rurality” for purposes of health-care planning: an empirical measure for Ontario. Ont Med Rev 67(33):52
Matheson FI, Dunn JR, Smith KLW et al (2012) Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health 103:S12-16
Reid RJ, Roos NP, MacWilliam L et al (2002) Assessing population health care need using a claims-based ACG morbidity measure: a validation analysis in the Province of Manitoba. Health Serv Res 37(1345):1364. https://doi.org/10.1111/1475-6773.01029
Weiner JP, Starfield BH, Steinwachs DM, Mumford LM (1991) Development and application of a population-oriented measure of ambulatory care case-mix. Med Care 29:452–472. https://doi.org/10.1097/00005650-199105000-00006
Mahar AL, Jeong Y, Zagorski B, Coburn N (2018) Validating an algorithm to identify metastatic gastric cancer in the absence of routinely collected TNM staging data. BMC Health Serv Res 18:309. https://doi.org/10.1186/s12913-018-3125-7
Austin PC (2009) Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat-Simul Comput 38:1228–1234
Mamdani M, Sykora K, Li P et al (2005) Reader’s guide to critical appraisal of cohort studies: 2 Assessing potential for confounding. BMJ 330:960–962. https://doi.org/10.1136/bmj.330.7497.960
Dignam JJ, Kocherginsky MN (2008) Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol 26:4027–4034. https://doi.org/10.1200/JCO.2007.12.9866
Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154
Raison CL, Miller AH (2003) Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry 54:283–294. https://doi.org/10.1016/s0006-3223(03)00413-x
McDaniel JS, Musselman DL, Porter MR et al (1995) Depression in patients with cancer. Diagnosis, biology, and treatment. Arch Gen Psychiatry 52:89–99. https://doi.org/10.1001/archpsyc.1995.03950140007002
Chiruvella A, Kooby DA (2016) Surgical management of pancreatic neuroendocrine tumors. Surg Oncol Clin N Am 25:401–421. https://doi.org/10.1016/j.soc.2015.12.002
Mirakhur B, Pavel ME, Pommier RF, et al (2018) Biochemical responses in symptomatic and asymptomatic patients with neuroendocrine tumors: pooled analysis of 2 phase 3 trials. Endocr Pract. https://doi.org/10.4158/EP-2018-0296
Bender DA (1983) Biochemistry of tryptophan in health and disease. Mol Aspects Med 6:101–197. https://doi.org/10.1016/0098-2997(83)90005-5
Hallet J, Davis LE, Isenberg-Grzeda E et al (2020) Gaps in the management of depression symptoms following cancer diagnosis: a population-based analysis of prospective patient-reported outcomes. Oncologist 25:e1098–e1108. https://doi.org/10.1634/theoncologist.2019-0709
Hallet J, Davis LE, Mahar AL, (10, et al (2019) Patterns of symptoms burden in neuroendocrine tumors: a population-based analysis of prospective patient-reported outcomes. Oncologist 24:1384–1394. https://doi.org/10.1634/theoncologist.2019-0112
Holland JC, Bultz BD, National Comprehensive Cancer Network (NCCN) (2007) the NCCN guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw 5:3–7
Bultz BD, Groff SL, Fitch M et al (2011) Implementing screening for distress, the 6th vital sign: a Canadian strategy for changing practice. Psychooncology 20(463):469. https://doi.org/10.1002/pon.1932
Davis LE, Bogner E, Coburn NG et al (2020) Stage at diagnosis and survival in patients with cancer and a pre-existing mental illness: a meta-analysis. J Epidemiol Community Health 74:84–94. https://doi.org/10.1136/jech-2019-212311
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). This study also received funding from the Canadian Institutes of Health Research (CIHR) and the North American Neuroendocrine Society (NANETS) New Clinical Investigator Scholarship. Parts of this material are based on data and information compiled and/or information provided by: Cancer Care Ontario (CCO) and CIHI. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
The datasets from this study are held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the datasets publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The dataset creation plan and underlying analytic code are available from the authors upon reasonable request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Funding
This work was supported by the NANETS New Clinical Investigator Scholarship and an operating grant from the Canadian Institute of Health Research (FRN #407301).
Author information
Authors and Affiliations
Contributions
Concept and study design: JH, EIG, CL, SS, SDM, and ALM. Data acquisition, analysis, and interpretation: JH, EIG, CL, VB, JZ, SS, SDM, AA, WCC, NGC, and ALM. Funding and administration: JH, EIG, CL, VB, NGC, and ALM. Manuscript draft: JH and ALM. Manuscript critical revision and final approval: JH, EIG, CL, VB, JZ, SS, SDM, AA, WCC, NGC, and ALM.
Corresponding author
Ethics declarations
Competing interests
JH has received speaking honoraria from Ipsen Biopharmaceuticals and Advanced Accelerator Applications. EIG has a consulting agreement with Celgene USA CL has received speaking honoraria from Ipsen Biopharmaceuticals and Novartis Oncology. SS has received speaking honoraria from Ipsen Biopharmaceuticals Canada and Novartis Oncology, and research grants from Novartis Oncology and EMD Serono. NC receives salary support from Cancer Care Ontario as Lead for Patient-Reported Outcomes, and an honorarium from Astra-Zeneca. All remaining authors have declared no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Implications for practice
Sub-groups of small bowel and lung neuroendocrine tumors (NETs) and of serotonin-producing NETs have a higher incidence of psychiatric illness than other cancers. It is critical to provide patients with appropriate and tailored psycho-social supportive care. Particular attention should be paid to patients with lung and small bowel NETs, and those with functional NETs, regarding symptoms indicating psychiatric illness or distress. Routine screening of patient-reported outcomes, as implemented in various cancer centres, can be used to do so.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hallet, J., Isenberg-Grzeda, E., Law, C.H.L. et al. Incidence of psychiatric illness in patients with neuroendocrine tumors: a comparative population-based analysis. Support Care Cancer 30, 9635–9646 (2022). https://doi.org/10.1007/s00520-022-07365-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07365-z