Abstract
Objective
To evaluate the correlation between sarcopenia and inflammation- and nutrition-based markers in metastatic colorectal cancer (mCRC) patients.
Materials and methods
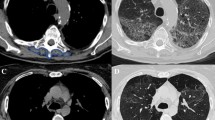
Age, body mass index (BMI), neutrophil/lymphocyte ratio (NLR), modified Glasgow prognostic score (mGPS), prognostic nutrition index (PNI), cachexia index (CIn), skeletal muscle index (SMI), controlling nutritional status (CONUT) score, and geriatric nutritional risk index (GNRI) were evaluated in 185 patients. Ideal cut-off values for the GNRI score were determined with the ROC curve analysis, and the patients were divided into two groups as low and high GNRI. Sarcopenia was diagnosed using CT scanning, the gold standard method. Univariate and multivariate Cox proportional hazard analyses were done based on the above-listed parameters to assess the correlation between sarcopenia and changes in immuno-nutrition and inflammatory response. Kaplan–Meier analysis was also done to evaluate survival.
Results
Univariate analysis of the 185 patients based on the EGWSOP 2018 threshold values showed correlation between the presence of sarcopenia and male gender, diagnosed colon cancer, history of metastasectomy, BMI < 24, high mGPS score, PNI score ≥ 45, high CONUT score, and low GNRI score (p < 0.05). In multivariate analysis, low GNRI (HR: 2.40; 95% CI: 1.03–5.544; p = 0.040), and high-CONUT scores (HR: 2.01; 95% CI: 1.06–3.73; p = 0.029) were identified as independent prognostic factors for the presence of sarcopenia.
Conclusion
GNRI and CONUT scores are elementary and practical predictors for sarcopenia, a condition which is associated with poor outcomes in mCRC patients.

Similar content being viewed by others
Data availability
The authors confirm that the data supportting the findings of this study are available within the article. Row data that supportting the findings of this study are available from the corresponding author, upon reasonable request.
References
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S-991S. https://doi.org/10.1093/jn/127.5.990S
Fuggle N, Shaw S, Dennison E, Cooper C (2017) Sarcopenia. Best Pract Res Clin Rheumatol 31:218–242. https://doi.org/10.1016/j.berh.2017.11.007
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635. https://doi.org/10.1016/S1470-2045(08)70153-0
Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC (2009) Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15:6973–6979. https://doi.org/10.1158/1078-0432.CCR-09-1525
Narici MV, Maffulli N (2010) Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95:139–159. https://doi.org/10.1093/bmb/ldq008
Bozzetti F (2017) Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 28:21072118. https://doi.org/10.1093/annonc/mdx271
Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S (2011) Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 18:3579–3585. https://doi.org/10.1245/s10434-011-1976-9
Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A, Yamanaka T (2013) Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 100:1523–1530. https://doi.org/10.1002/bjs.9258
Heymsfield SB, Wang Z, Baumgartner RN, Ross R (1997) Human body composition: advances in models and methods. Annu Rev Nutr 17:527–558. https://doi.org/10.1146/annurev.nutr.17.1.527
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D (1985) Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122. https://doi.org/10.1152/jappl.1998.85.1.115
Kodera Y (2015) More than 6 months of postoperative adjuvant chemotherapy results in loss of skeletal muscle: a challenge to the current standard of care. Gastric Cancer 18:203–204. https://doi.org/10.1007/s10120-014-0381-z
Coelen RJS, van Gulik TM (2015) Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg 39:2368–2369. https://doi.org/10.1007/s00268-015-3053-1
Jung HW, Kim JW, Kim JY, Kim SW, Yang HK, Lee JW, Lee KW, Kim DW, Kang SB, Kim KI, Kim CH, Kim JH (2015) Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer 23(3):687–694. https://doi.org/10.1007/s00520-014-2418-6
Luo H, Chen Y, Chuang Y, Cheng YT, Lee WC, Kang CH, Chiang PH (2014) Subclassification of upper urinary tract urothelial carcinoma by the neutrophil-to-lymphocyte ratio (NLR) improves prediction of oncological outcome. BJU Int 113:144–149. https://doi.org/10.1111/bju.12582
Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G (2005) CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20:38–45 (PMID: 15762418)
Pan QX, Su ZJ, Zhang JH, Wang CR, Ke SY (2015) A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. OncoTargets Ther 8:1375–1385. https://doi.org/10.2147/OTT.S82437
Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, Baba Y, Sakamoto Y, Yamashita Y, Yoshida N, Chikamoto A, Baba H (2018) Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 21:204–212. https://doi.org/10.1007/s10120-017-0744-3
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2018) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. https://doi.org/10.1093/ageing/afy169
Hasselmann M, Alix E (2003) Tools and procedures for screening for malnutrition and its associated in risks in hospital. Nutr Clin Metabol 17:218–226. https://doi.org/10.1016/j.nupar.2003.09.004
Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF (2009) Estimation of cachexia among cancer patients based on four definitions. J Oncol 1-7. https://doi.org/10.1155/2009/693458
Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S (2001) Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 18:3579–3585. https://doi.org/10.1245/s10434-011-1976-9
Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A, Yamanaka T (2013) Sarcopenia as a predictor of prognosis in patients following hepatectomy. Br J Surg 100:1523–1530. https://doi.org/10.1002/bjs.9258
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67. https://doi.org/10.1016/j.ejca.2015.12.:26882087030
Roxburgh CS, Platt JJ, Leitch EF, Kinsella J, Horgan PG, McMillan DC (2011) Relationship between preoperative comorbidity, systemic inflammatory response, and survival in patients undergoing curative resection for colorectal cancer. Ann Surg Oncol 18:997–1005. https://doi.org/10.1245/s10434-010-1410-8 (PMID: 21042941)
Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, Zhou CJ, Shen X, Yu Z (2015) Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis 17:O256–O264. https://doi.org/10.1111/codi.13067
Huang DD, Chen XX, Chen XY, Wang SL, Shen X, Chen XL, Yu Z, Zhuang CL (2016) Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol 142:2347–2356. https://doi.org/10.1007/s00432-016-2230-4
Kazemi-Bajestani SMR, Mazurak VC, Baracos V (2016) Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 54:2–10. https://doi.org/10.1016/j.semcdb.2015.09.001
Lorent JH, Léonard C, Abouzi M, Akabi F, Quetin-Leclercq J, Mingeot-Leclercq MP (2016) α-Hederin induces apoptosis, membrane permeabilization and morphologic changes in two cancer cell lines through a cholesterol-dependent mechanism. Planta Med 82:1532–1539. https://doi.org/10.1055/s-0042-114780
Lim JA, Oh CS, Yoon TG, Lee JY, Lee SH, Yoo YB, Yang JH, Kim SH (2018) The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer 18:159. https://doi.org/10.1186/s12885-018-4064-8
Lucijanic M, Veletic I, Rahelic D, Pejsa V, Cicic D, Skelin M, Livun A, Tupek KM, Stoos-Veic T, Lucijanic T, Maglicic A, Kusec R (2018) Assessing serum albumin concentration, lymphocyte count and prognostic nutritional index might improve prognostication in patients with myelofibrosis. Wien Klin Wochenschr 130:126–133. https://doi.org/10.1007/s00508-018-1318-z
Jeon CH, Park KB, Jung YJ, Seo HS, Park CH, Song KY, Lee HH (2020) Modified controlling nutritional status score: a refined prognostic indicator depending on the stage of gastric cancer. Surg Oncol 34:261–269. https://doi.org/10.1016/j.suronc.2020.05.008
Wei Y, Xu H, Dai J, Peng J, Wang W, Xia L, Zhou F (2018) Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. Biomed Res Int 2018:1804086. https://doi.org/10.1155/2018/1804086PMID:30627541;PMCID:PMC6304480
Costa MD, Vieira de Melo CY, Amorim AC, Cipriano Torres Dde O, Dos Santos AC (2016) Association between nutritional status, inflammatory condition, and prognostic indexes with postoperative complications and clinical outcome of patients with gastrointestinal neoplasia. Nutr Cancer 68:1108–1114. https://doi.org/10.1080/01635581.2016.1206578
Mohamed Sad L, Elsaka AM, Abdelmonem Zamzam Y, Gharib Khairallah F (2010) Phase angle, body mass index and KRAS status of metastatic colorectal cancer in response to chemotherapy with and without target therapy: clinical impact and survival. J BUON 25:914–926 (PMID: 32521886)
Cybulska-Stopa B, Ługowska I, Wiśniowski R, Domagała-Haduch M, Rajczykowski M, Piejko K, Bar-Letkiewicz I, Suwiński R, Regulski K, Mackiewicz J (2020) Overweight is associated with better prognosis in metastatic colorectal cancer patients treated with bevacizumab plus FOLFOX chemotherapy. Contemp Oncol (Pozn) 24:34–41. https://doi.org/10.5114/wo.2020.94728
Aparicio T, Ducreux M, Faroux R, for FFCD investigators, et al (2018) Overweight is associated to a better prognosis in metastatic colorectal cancer: a pooled analysis of FFCD trials. Eur J Cancer 98:1–9. https://doi.org/10.1016/j.ejca.2018.03.031
Lidoriki I, Schizas D, Frountzas M, Machairas N, Prodromidou A, Kapelouzou A, Karavokyros I, Pikoulis E, Kales SN, Liakakos T (2021) GNRI as a prognostic factor for outcomes in cancer patients: a systematic review of the literature. Nutr Cancer 73:391–403. https://doi.org/10.1080/01635581.2020.1756350
Lee GW, Go SI, Kim DW, Kim HG, Kim JH, An HJ, Jang JS, Kim BS, Hahn S, Heo DS (2020) Geriatric Nutritional Risk Index as a prognostic marker in patients with extensive-stage disease small cell lung cancer: results from a randomized controlled trial. Thorac Cancer 11:62–71. https://doi.org/10.1111/1759-7714.13229
Okamoto T, Hatakeyama S, Narita S, Takahashi M, Sakurai T, Kawamura S et al (2019) Impact of nutritional status on the prognosis of patients with metastatic hormone-naive prostate cancer: a multicenter retrospective cohort study in Japan. World J Urol 37:1827–1835. https://doi.org/10.1007/s00345-018-2590-2
Nakayama M, Gosho M, Adachi M, Ii R, Matsumoto S, Miyamoto H, Hirose Y, Nishimura B, Tanaka S, Wada T, Tabuchi K (2021) The Geriatric Nutritional Risk Index as a prognostic factor in patients with advanced head and neck cancer. Laryngoscope 131:E151–E156. https://doi.org/10.1002/lary.28587
Migita K, Matsumoto S, Wakatsuki K, Ito M, Kunishige T, Nakade H, Sho M (2018) The prognostic significance of the Geriatric Nutritional Risk Index in patients with esophageal squamous cell carcinoma. Nutr Cancer 70:1237–1245. https://doi.org/10.1080/01635581.2018.1512640
Sugawara K, Yamashita H, Urabe M, Okumura Y, Yagi K, Aikou S, Seto Y (2021) Geriatric Nutrition Index influences survival outcomes in gastric carcinoma patients undergoing radical surgery. JPEN J Parenter Enteral Nutr 45:1042–1051. https://doi.org/10.1002/jpen.1978
Bahat G, Tufan A, Tufan F, Kilic C, Akpinar TS, Kose M, Erten N, Karan MA, Cruz-Jentoft AJ (2016) Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr 35:1557–1563. https://doi.org/10.1016/j.clnu.2016.02.002
Author information
Authors and Affiliations
Contributions
Study concept: ZGG, TY. Study design: ZGG, TY. Data acquisition: ZGG, HAÖ, CA. Quality control of data: ZGG, HAÖ, CA. Data analysis and interpretation: ZGG, HAÖ, CA. Statistical analysis: ZGG, HE. Manuscript editing: ZGG, TY. Manuscript review: ZGG, TY.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board at the Izmir Dokuz Eylül University.
Consent to participate
All patients provided written informed consent to participate in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Güç, Z.G., Altay, C., Özgül, H.A. et al. GNRI And Conut Scores: Simple Predictors of Sarcopenia in Metastatic Colorectal Cancer Patients. Support Care Cancer 30, 7845–7852 (2022). https://doi.org/10.1007/s00520-022-07218-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07218-9