Abstract
Purpose
This study explored whether symptom relief differs by sex in patients with cancer receiving medical cannabis (MC) therapy.
Methods
This is an analysis of data collected from patients with cancer enrolled in the Quebec Cannabis Registry. MC was initiated for the therapeutic management of cancer symptoms. Patients completed the revised Edmonton Symptom Assessment System (ESAS-r) questionnaire at baseline and 3-month follow-up. We examined the interaction between sex and time on each ESAS-r symptom and the interaction between time and tetrahydrocannabinol:cannabidiol (THC:CBD) ratios for each sex on total symptom burden.
Results
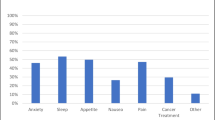
The analysis included 358 patients (M: 171). There were no sex differences in baseline ESAS-r scores. Three months of MC therapy led to significant improvements in pain (M: − 1.4 ± 0.3, p < 0.001; F: − 1.1 ± 0.3, p < 0.01), tiredness (M: − 1.7 ± 0.4, p < 0.001; F: − 1.2 ± 0.4, p < 0.05), anxiety (M: − 1.1 ± 0.4, p < 0.05; F: − 1.2 ± 0.4, p < 0.001), and well-being (M: − 1.2 ± 0.4, p < 0.05; F: − 1.4 ± 0.4, p < 0.01) in both sexes. Only F perceived improved drowsiness (− 1.1 ± 0.4, p < 0.05), nausea (− 0.9 ± 0.3, p < 0.05), lack of appetite (− 1.7 ± 0.4, p < 0.001), and shortness of breath (− 0.9 ± 0.3, p < 0.05). From baseline to 3-month follow-up, THC-dominant MC significantly reduced pain (− 1.52 ± 0.52, p < 0.05) in M, whereas in F it diminished nausea (− 2.52 ± 0.70, p < 0.01) and improved well-being (− 2.41 ± 0.79, p < 0.05). THC:CBD-balanced products significantly reduced pain (− 1.48 ± 0.49, p < 0.05), tiredness (− 1.82 ± 0.62, p < 0.05), anxiety (− 1.83 ± 0.54, p < 0.05), and improved well-being (− 2.01 ± 0.56, p < 0.01) in M. CBD-dominant products did not offer significant symptom relief in either sex.
Conclusion
The perceived relief of cancer symptoms from MC differs between sexes. More randomized controlled trials are needed to confirm our findings.




Similar content being viewed by others
Data availability
Data may be made available from the corresponding author upon request.
Code availability
N/A.
References
Olver I, Keefe D, Herrstedt J, Warr D, Roila F, Ripamonti CI (2020) Supportive care in cancer—a MASCC perspective. Support Care Cancer 28:3467–3475
Arboleda MF, Prosk E, Cyr C, Gamaoun R, Vigano A (2020) Medical cannabis in supportive cancer care: Lessons from Canada. Support Care Cancer 14:1–3
Birdsall SM, Birdsall TC, Tims LA (2016) The use of medical marijuana in cancer. Curr Oncol Rep 18:40
Cyr C, Arboleda MF, Aggarwal SK, Balneaves LG, Daeninck P, Néron A, Prosk E, Vigano A (2018) Cannabis in palliative care: current challenges and practical recommendations. Ann Palliat Med 7:463–477
Martell K, Fairchild A, LeGerrier B, Sinha R, Baker S, Liu H, Ghose A, Olivotto I, Kerba M (2018) Rates of cannabis use in patients with cancer. Curr Oncol 25:219–225
Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT (2010) Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 39:167–179
Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT (2013) An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage 46:207–218
Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, Wright S, Kornyeyeva E, Fallon MT (2018) Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manage 55(179–188):e171
Chang YD, Jung J-W, Oberoi-Jassal R, Kim J, Rajasekhara S, Haas M, Smith J, Desai V, Donovan KA, Portman D (2019) Edmonton Symptom Assessment Scale and clinical characteristics associated with cannabinoid use in oncology supportive care outpatients. J Natl Compr Cancer Netw 17:1059–1064
Vigano A, Aprikian S, Kasvis P, Bacis V, Al Harrasi A, Aubin NM, Vigano M, Borod M (2020) Safety and effectiveness of medical cannabis as a complementary option for supportive cancer care: results from the Cannabis Pilot Project. J Clin Oncol 38:12106
Brisbois T, De Kock I, Watanabe S, Mirhosseini M, Lamoureux D, Chasen M, MacDonald N, Baracos V, Wismer W (2011) Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol 22:2086–2093
Duran M, Pérez E, Abanades S, Vidal X, Saura C, Majem M, Arriola E, Rabanal M, Pastor A, Farré M (2010) Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br J Clin Pharmacol 70:656–663
Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang H-M, Baranowski V (2007) Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin 23:533–543
Walsh D, Donnelly S, Rybicki L (2000) The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 8:175–179
Miaskowski C (2004) Gender differences in pain, fatigue, and depression in patients with cancer. J Natl Cancer Inst Monogr 2004:139–143
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7:6–9
Hui D, Bruera E (2017) The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage 53:630–643
Hui D, Shamieh O, Paiva CE, Perez-Cruz PE, Kwon JH, Muckaden MA, Park M, Yennu S, Kang JH, Bruera E (2015) Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: a prospective, multicenter study. Cancer 121:3027–3035
Bar-Sela G, Vorobeichik M, Drawsheh S, Omer A, Goldberg V, Muller E (2013) The medical necessity for medicinal cannabis: prospective, observational study evaluating the treatment in cancer patients on supportive or palliative care. Evid Based Complement Alternat Med 2013:510392
Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko Y-D, Schnelle M, Reif M, Cerny T (2006) Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 24:3394–3400
Bar-Lev Schleider L, Mechoulam R, Lederman V, Hilou M, Lencovsky O, Betzalel O, Shbiro L, Novack V (2018) Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med 49:37–43
Redmond WJ, Goffaux P, Potvin S, Marchand S (2008) Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin 24:1017–1024
Cooper ZD, Haney M (2014) Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend 136:85–91
Calakos KC, Bhatt S, Foster DW, Cosgrove KP (2017) Mechanisms underlying sex differences in cannabis use. Curr Addict Rep 4:439–453
Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, Bormans G (2008) Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F] MK-9470 PET. Neuroimage 39:1533–1541
Laurikainen H, Tuominen L, Tikka M, Merisaari H, Armio R-L, Sormunen E, Borgan F, Veronese M, Howes O, Haaparanta-Solin M (2019) Sex difference in brain CB1 receptor availability in man. Neuroimage 184:834–842
Normandin MD, Zheng M-Q, Lin K-S, Mason NS, Lin S-F, Ropchan J, Labaree D, Henry S, Williams WA, Carson RE (2015) Imaging the cannabinoid CB1 receptor in humans with [11C] OMAR: assessment of kinetic analysis methods, test–retest reproducibility, and gender differences. J Cereb Blood Flow Metab 35:1313–1322
Nia AB, Mann C, Kaur H, Ranganathan M (2018) Cannabis use: neurobiological, behavioral, and sex/gender considerations. Curr Behav Neurosci Rep 5:271–280
Craft RM, Marusich JA, Wiley JL (2013) Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci 92:476–481
Blanton HL, Barnes RC, McHann MC, Bilbrey JA, Wilkerson JL, Guindon J (2021) Sex differences and the endocannabinoid system in pain. Pharmacol Biochem Behav. 173107
Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, Olver I, Walsh A, McGregor I, Cheung Y (2020) Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting (CINV): a randomised, placebo-controlled, phase 2 crossover trial. Ann Oncol 31:1553–1560
Aviram J, Lewitus GM, Vysotski Y, Uribayev A, Procaccia S, Cohen I, Leibovici A, Abo-Amna M, Akria L, Goncharov D (2020) Short-term medical cannabis treatment regimens produced beneficial effects among palliative cancer patients. Pharmaceuticals 13:435
Hardy J, Haywood A, Gogna G, Martin J, Yates P, Greer R, Good P (2020) Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo-controlled, randomised clinical trial of efficacy and safety of 1:1 delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Trials 21:1–8
Good P, Haywood A, Gogna G, Martin J, Yates P, Greer R, Hardy J (2019) Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD). BMC Palliat Care 18:110
Acknowledgements
The authors would like to acknowledge Dr. Robert Kilgour for his contribution to the final review of this manuscript. We would also like the acknowledge the support of the Quebec Cannabis Registry scientific committee members (Yola Moride, Marc O. Martel, Jordi Perez, Andrée Néron, Pierre Beaulieu, and Julie Desroches) and thank Dr. Mark Ware, who initiated the QCR in 2015 and was the Principal Investigator until July 1, 2018. Finally, we would like to thank the Cedars Cancer Foundation and Rossy Cancer Network for supporting the Medical Cannabis Program in Oncology of the McGill University Health Center, which provided most data on oncology patients to the QCR.
Funding
The QCR was supported by grants from the Canadian Consortium for the Investigation of Cannabinoids (CCIC), the Collège des Médecins du Québec (CMQ), and unrestricted grants from several licensed cannabis producers (Bedrocan, Mettrum, and Tweed; these three companies merged during the study conduct into one company called Canopy Growth Corporation). Cedars Cancer Foundation and Rossy Cancer Network supported the Medical Cannabis Program in Oncology of the McGill University Health Center, which provided most data on oncology patients to the QCR. No funding agency had input into the design and conduct of the QCR, although the CMQ was present at development meetings.
Author information
Authors and Affiliations
Contributions
P. K.: conceptualization, formal analysis, writing-original draft, review, and editing. M. C. M.: project manager of the Quebec Cannabis Registry, data management, manuscript review, and editing. S. A. and M. L. V.: data management, manuscript review and editing. A. V.: conceptualization, manuscript review and editing, responsible for the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval for the QCR was granted by the McGill University Health Center Research Ethics Board.
Consent to participate
All patients provided informed consent prior to participating in the QCR.
Consent for publication
All patients provided informed consent to the eventual publication of results from the data collected, prior to participating in the QCR.
Competing interests
S. A. holds stock options in a licensed medical cannabis producer (Tilray); A. V. sat on the medical advisory boards of licensed producers of medical cannabis in Canada (Spectrum Therapeutics, Tilray, Syqe, and EmpowerPharm) and is the former research director for Santé Cannabis, Montreal, Canada. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kasvis, P., Canac-Marquis, M., Aprikian, S. et al. Sex differences exist in the perceived relief of cancer symptoms with medical cannabis: results from the Quebec Cannabis Registry. Support Care Cancer 30, 7863–7871 (2022). https://doi.org/10.1007/s00520-022-07193-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07193-1