Abstract
Purpose
Mucositis is severely painful and often reported as one of the most distressing adverse effects of cancer therapy; it is a significant threat to quality of life as well as life itself. Anti-inflammatory agents may modulate physiologic mechanisms that perpetuate mucositis and be useful in prevention efforts. Because systemic anti-inflammatory agents are not appropriate for many patients, locally acting agents (mouthwashes) may be more feasible for use. This review and meta-analysis evaluates the role that anti-inflammatory mouthwashes have in preventing or reducing oral mucositis associated with chemotherapy and radiation therapy.
Methods
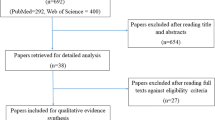
A systematic literature review was conducted to identify studies evaluating the efficacy of anti-inflammatory mouthwashes to prevent therapy-associated mucositis. Meta-analysis was conducted to determine efficacy in preventing any mucositis and dose-limiting mucositis.
Results
Eight peer-reviewed publications were identified; corticosteroid and nonsteroidal anti-inflammatory mouthwashes are effective in reducing overall incidence of mucositis and are associated with lower severity of mucositis. Meta-analysis reveals significant reduction in symptomatic mucositis incidence (OR 6.00, 95% CI 4.39–8.20, p < 0.0001) and reduction of dose-limiting mucositis (OR 2.12, 95% CI 1.07–4.28, p = 0.032).
Conclusion
Mouthwashes containing anti-inflammatory agents are a potential effective means to prevent or reduce mucositis associated with cancer therapy. There are limited adverse effects from these agents, and adherence is high, indicating safety and feasibility of use. Anti-inflammatory mouthwashes should be considered for supportive care in persons at risk for mucositis and must be further evaluated to investigate efficacy across multiple chemotherapy agents, adverse effects, and impacts on symptoms, pain, and quality of life.

Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Guimarães J, Carvalho L, Damascena L et al (2021) The incidence of severe oral mucositis and its occurrence sites in pediatric oncologic patients. Med Oral Patol Oral Cir Bucal 26(3):e299–e303. https://doi.org/10.4317/medoral.24185
Çakmak S, Nural N (2019) Incidence of and risk factors for development of oral mucositis in outpatients undergoing cancer chemotherapy. Int J Nurs Pract 25(1):e12710. https://doi.org/10.1111/ijn.12710
Gabriel AF, Silveira FM, Curra M, et al Risk factors associated with the development of oral mucositis in pediatric oncology patients: systematic review and meta-analysis. Oral Dis https://doi.org/10.1111/odi.13863
McCullough RW (2017) US oncology-wide incidence, duration, costs and deaths from chemoradiation mucositis and antimucositis therapy benefits. Future Oncol 13(30):2823–2852. https://doi.org/10.2217/fon-2017-0418
Pulito C, Cristaudo A, Porta CL et al (2020) Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 39(1):210. https://doi.org/10.1186/s13046-020-01715-7
Lalla RV, Brennan MT, Gordon SM, Sonis ST, Rosenthal DI, Keefe DM. Oral mucositis due to high-dose chemotherapy and/or head and neck radiation therapy. J Natl Cancer Inst Monogr 2019;2019(53). https://doi.org/10.1093/jncimonographs/lgz011
Otmani N, Hattad S (2021) Clinical outcome in children with chemotherapy-induced mucositis. Semin Oncol Nurs 37(3):151160. https://doi.org/10.1016/j.soncn.2021.151160
Shu Z, Zeng Z, Yu B et al (2020) Nutritional status and its association with radiation-induced oral mucositis in patients with nasopharyngeal carcinoma during radiotherapy: a prospective study. Front Oncol. https://doi.org/10.3389/fonc.2020.594687
Sobue T, Bertolini M, Thompson A, Peterson DE, Diaz PI, Dongari-Bagtzoglou A (2018) Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol Oral Microbiol 33(3):212–223. https://doi.org/10.1111/omi.12214
Kishimoto M, Akashi M, Tsuji K et al (2017) Intensity and duration of neutropenia relates to the development of oral mucositis but not odontogenic infection during chemotherapy for hematological malignancy. PLoS ONE 12(7):e0182021. https://doi.org/10.1371/journal.pone.0182021
Kanagalingam J, Wahid M, Lin J et al (2018) Patient and oncologist perceptions regarding symptoms and impact on quality-of-life of oral mucositis in cancer treatment: results from the awareness drives oral mucositis PercepTion (ADOPT) study. Support Care Cancer 26(7):2191–2200. https://doi.org/10.1007/s00520-018-4050-3
Hamouda N, Sano T, Oikawa Y et al (2017) Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic Clin Pharmacol Toxicol 121(3):159–168. https://doi.org/10.1111/bcpt.12793
Basile D, Di Nardo P, Corvaja C et al (2019) Mucosal injury during anti-cancer treatment: from pathobiology to bedside. Cancers 11(6):857. https://doi.org/10.3390/cancers11060857
Wong HM 2014 Oral complications and management strategies for patients undergoing cancer therapy. Sci World J 2014:581795https://doi.org/10.1155/2014/581795
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4(4):277–284. https://doi.org/10.1038/nrc1318
Al-Ansari S, Zecha JAEM, Barasch A, de Lange J, Rozema FR, Raber-Durlacher J (2015) Oral mucositis induced by anticancer therapies. Curr Oral Health Rep 2(4):202–211. https://doi.org/10.1007/s40496-015-0069-4
Ariyawardana A, Cheng K, Kandwal A et al (2019) Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 27(10):3985–3995. https://doi.org/10.1007/s00520-019-04888-w
Thomsen M, Vitetta L (2018) Adjunctive treatments for the prevention of chemotherapy- and radiotherapy-induced mucositis. Integr Cancer Ther 17(4):1027–1047. https://doi.org/10.1177/1534735418794885
Mahendran VJ, Stringer AM, Semple SJ, Song Y, Garg S (2018) Advances in the use of anti-inflammatory agents to manage chemotherapy-induced oral and gastrointestinal mucositis. Curr Pharm Des 24(14):1518–1532. https://doi.org/10.2174/1381612824666180409093918
Shankar A, Roy S, Bhandari M et al (2017) Current trends in management of oral mucositis in cancer treatment. Asian Pac J Cancer Prev 18(8):2019–2026. https://doi.org/10.22034/APJCP.2017.18.8.2019
Elad S, Cheng KKF, Lalla RV et al (2020) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126(19):4423–4431. https://doi.org/10.1002/cncr.33100
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 18(3):e1003583. https://doi.org/10.1371/journal.pmed.1003583
Dang D, Dearholt SL, Bissett K, Ascenzi J, Whalen M (2021) Johns Hopkins evidence-based practice for nurses and healthcare professionals, fourth edition. 4th ed. Sigma
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introducetion - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Biostat Inc. Comprehensive meta-analysis. Comprehensive Meta-Analysis Web site. https://www.meta-analysis.com/. Updated 2019. Accessed 9/9/, 2021.
Hattori M, Hagiwara S, Kotani H et al (2019) A single-arm, phase 2 study of steroid-containing mouthwash for the prevention of everolimus-associated stomatitis in multiple tumor types. Int J Clin Oncol 24(10):1320–1327. https://doi.org/10.1007/s10147-019-01476-0
Jones VE, Mcintyre KJ, Paul D et al (2019) Evaluation of miracle mouthwash plus hydrocortisone versus prednisolone mouth rinses as prophylaxis for everolimus-associated stomatitis: a randomized phase II study. Oncol 24(9):1153. https://doi.org/10.1634/theoncologist.2018-0340
Rugo HS, Seneviratne L, Beck JT et al (2017) Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol 18(5):654–662. https://doi.org/10.1016/S1470-2045(17)30109-2
Rastogi M, Khurana R, Revannasiddaiah S et al (2017) Role of benzydamine hydrochloride in the prevention of oral mucositis in head and neck cancer patients treated with radiotherapy (>50 Gy) with or without chemotherapy. Support Care Cancer 25(5):1439–1443. https://doi.org/10.1007/s00520-016-3548-9
Epstein JB, Silverman DS et al (2001) Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 92(4):875–885. https://doi.org/10.1002/1097-0142(20010815)92:4%3c875::aid-cncr1396%3e3.0.co;2-1
Sheibani KM, Mafi AR, Moghaddam S, Taslimi F, Amiran A, Ameri A (2015) Efficacy of benzydamine oral rinse in prevention and management of radiation-induced oral mucositis: a double-blind placebo-controlled randomized clinical trial. Asia Pac J Clin Oncol 11(1):22–27. https://doi.org/10.1111/ajco.12288
Kazemian A, Kamian S, Aghili M, Hashemi FA, Haddad P (2009) Benzydamine for prophylaxis of radiation-induced oral mucositis in head and neck cancers: a double-blind placebo-controlled randomized clinical trial. Eur J Cancer Care 18(2):174–178. https://doi.org/10.1111/j.1365-2354.2008.00943.x
Chitapanarux I, Tungkasamit T, Petsuksiri J et al (2017) Randomized control trial of benzydamine HCl versus sodium bicarbonate for prophylaxis of concurrent chemoradiation-induced oral mucositis. Support Care Cancer 26(3):879. https://doi.org/10.1007/s00520-017-3904-4
Kiran MS, Vidya S, Aswal GS, Kumar V, Rai V (2017) Systemic and topical steroids in the management of oral mucosal lesions. J Pharm Bioallied Sci 9:S1–S3. https://doi.org/10.4103/jpbs.JPBS_91_17
Savage NW, McCullough MJ (2005) Topical corticosteroids in dental practice. Aust Dent J 50(4 Suppl 2):40. https://doi.org/10.1111/j.1834-7819.2005.tb00385
Zadik Y, Elad S, Shapira A, Shapira MY (2017) Treatment of oral mucosal manifestations of chronic graft-versus-host disease: dexamethasone vs. budesonide. Expert Opin Pharmacother 18(3):235–242. https://doi.org/10.1080/14656566.2017.1282464
Nicolatou-Galitis O, Bossi P, Orlandi E (2021) BensadounR-J 2021 The role of benzydamine in prevention and treatment of chemoradiotherapy-induced mucositis. Support Care 29(10):5701–5709. https://doi.org/10.1007/s00520-021-06048-5
AlQahtani RM, Abdalla M, Azzam YH, Elsherif, AA, Altulayhi RI. Pharmacological interventions for post-operative sore throat (POST): a network meta-analysis. 2021;17(1):169–177. https://doi.org/10.22514/sv.2020.16.0085
Sonis ST, Watkins B, Fey E, Yuschak M, Parenti D (2005) Mechanism of action of benzydamine in the treatment of oral mucositis. JCO 23(16):8040. https://doi.org/10.1200/jco.2005.23.16_suppl.8040
Golac-Guzina N, Novaković Z, Sarajlić Z et al (2019) Comparative study of the efficacy of the lysozyme, benzydamine and chlorhexidine oral spray in the treatment of acute tonsillopharyngitis - results of a pilot study. Acta Medica Academia 48(2):140–146. https://doi.org/10.5644/ama2006-124.252[doi]
Zheng Z, Zhao X, Zhao Q et al (2020) The effects of early nutritional intervention on oral mucositis and nutritional status of patients with head and neck cancer treated with radiotherapy. Front Oncol 10:595632. https://doi.org/10.3389/fonc.2020.595632
Alsheyyab F, Al-Momani D, Kasht R, Kamal A, Abusalem D, Al-Qasem W (2021) Impact of severe oral mucositis in pediatric cancer patients on resource utilization and cancer treatment plans. Int J Clin Pharm 43(5):1322–1326. https://doi.org/10.1007/s11096-021-01253-y
Orgel E, Sposto R, Malvar J et al (2014) Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol 32(13):1331–1337. https://doi.org/10.1200/JCO.2013.52.6962
Laviano A, Di Lazzaro L, Koverech A (2018) Nutrition support and clinical outcome in advanced cancer patients. Proc Nutr Soc 77(4):388–393. https://doi.org/10.1017/S0029665118000459
Kim SH, Lee SM, Jeung HC et al (2019) The effect of nutrition intervention with oral nutritional supplements on pancreatic and bile duct cancer patients undergoing chemotherapy. Nutrients 11(5):1145. https://doi.org/10.3390/nu11051145
Lin T, Yang J, Hong X, Yang Z, Ge T, Wang M (2020) Nutritional status in patients with advanced lung cancer undergoing chemotherapy: a prospective observational study. Nutr Cancer 72(7):1225–1230. https://doi.org/10.1080/01635581.2019.1675720
Bozzetti F (2017) Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 28(9):2107–2118. https://doi.org/10.1093/annonc/mdx271
Murphy CC, Fullington HM, Alvarez CA et al (2018) Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer 124(13):2850–2857. https://doi.org/10.1002/cncr.31389
Chen L, Trares K, Laetsch DC, Nguyen TNM, Brenner H, Schöttker B (2021) Systematic review and meta-analysis on the associations of polypharmacy and potentially inappropriate medication with adverse outcomes in older cancer patients. J Gerontol 76(6):1044–1052. https://doi.org/10.1093/gerona/glaa128
Calip GS, Xing S, Jun D, Lee W, Hoskins KF, Ko NY (2017) Polypharmacy and adherence to adjuvant endocrine therapy for breast cancer. J Oncol Pract 13(5):e451–e462. https://doi.org/10.1200/JOP.2016.018317
Plemons JM, Rees TD, Zachariah NY (1990) Absorption of a topical steroid and evaluation of adrenal suppression in patients with erosive lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol 69(6):688–693. https://doi.org/10.1016/0030-4220(90)90349-w
Diaz PI, Hong B, Dupuy AK et al (2019) Integrated analysis of clinical and microbiome risk factors associated with the development of oral candidiasis during cancer chemotherapy. J Fungi 5(2):49. https://doi.org/10.3390/jof5020049
Author information
Authors and Affiliations
Contributions
Dr. CT devised the idea of this work and directed the overall conduct of this review and meta-analysis, was responsible for constructing and evaluating the search strategy, executing the search strategy, and organizing the research team, and performed primary data extraction from articles and drafting the manuscript. Ms. ML was responsible for 2nd execution of the search strategy, conducting review of identified articles to verify they met inclusion criteria, performed adjudication with Mr. T on article inclusion, and extracted data for 2nd check of validity of findings as well as verified agreement with article level of evidence and quality; she assisted with drafting and writing sections of the manuscript. Dr. CB provided biostatistical expertise and oversight to data extraction, exploring data amenable to meta-analysis, and conduct of the biostatistical modeling for the meta-analysis as well as reviewing findings and interpretation of quantitative results. Dr. CHY and Dr. KR provided primary oversight of this project, verifying adequate search strategy, clinical relevance, and offering expertise in oncology care and symptom science; they both provided final revisions to the manuscript and mentoring for conduct of this project throughout execution.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thornton, C.P., Li, M., Budhathoki, C. et al. Anti-inflammatory mouthwashes for the prevention of oral mucositis in cancer therapy: an integrative review and meta-analysis. Support Care Cancer 30, 7205–7218 (2022). https://doi.org/10.1007/s00520-022-07068-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07068-5