Abstract
Purpose
Paracentesis is among the most widely utilized treatments for malignant ascites (MA). However, paracentesis in patients with MA has the potential to be associated with life-shortening effects. Thus, this study aimed to investigate whether paracentesis affected the duration of survival in such patients.
Methods
We performed a post hoc analysis of a prospective multicenter observational study investigating the dying process and end-of-life care in patients with terminal cancer, admitted to 23 palliative care units in Japan. Survival duration was compared between patients who did (paracentesis group) and did not undergo paracentesis (non-paracentesis group). We used inverse probability of treatment weighting (IPTW) to control for baseline covariates between groups.
Results
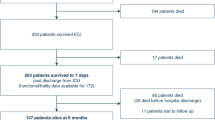
Among the 1896 initially enrolled patients, 568 with ascites were included in the study cohort. Eighty-five (15.0%) patients underwent paracentesis. The primary tumor site was the pancreas (51.9%, n = 295), followed by the gastrointestinal tract (22.7%, n = 129). Non-adjusted median durations of survival were 22 days (95% confidence interval [CI]: 16–25) and 12 days (95% CI: 11–13) in the paracentesis and non-paracentesis groups, respectively (hazard ratio [HR]: 0.69, 95% CI: 0.54–0.88; p = 0.003). The IPTW-adjusted median survival durations were 22 (95% CI: 16–25) and 16 days (95% CI: 12–22) in the paracentesis and non-paracentesis groups, respectively (HR: 0.89, 95% CI: 0.64–1.24; p = 0.492). No serious adverse events occurred in the paracentesis group.
Conclusions
Paracentesis does not negatively affect the survival of patients with cancer and MA and can be a standard treatment in palliative care settings.



Similar content being viewed by others
Data availability
The datasets generated during the current study are not publicly available due to ethical restrictions but are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Becker G, Galandi D, Blum HE (2006) Malignant ascites: systematic review and guideline for treatment. Eur J Cancer 42:589–597
Runyon BA (1994) Care of patients with ascites. N Engl J Med 330:337–342
Runyon BA (1994) Malignancy-related ascites and ascitic fluid “humoral tests of malignancy.” J Clin Gastroenterol 18:94–98
Wilailak S, Linasmita V, Srivannaboon S (1999) Malignant ascites in female patients: a seven-year review. J Med Assoc Thai 82:15–19
Appelqvist P, Silvo J, Salmela L et al (1982) On the treatment and prognosis of malignant ascites: is the survival time determined when the abdominal paracentesis is needed? J Surg Oncol 20:238–242
Ringenberg QS, Doll DC, Loy TS et al (1989) Malignant ascites of unknown origin. Cancer 64:753–755
Mackey JR, Venner PM (1996) Malignant ascites: demographics, therapeutic efficacy and predictors of survival. Can J Oncol 6:474–480
Garrison RN, Kaelin LD, Galloway RH et al (1986) Malignant ascites. Clinical and experimental observations Ann Surg 203:644–651
Lee CW, Bociek G, Faught W (1998) A survey of practice in management of malignant ascites. J Pain Symptom Manag 16:96–101
Chung M, Kozuch P (2008) Treatment of malignant ascites. Curr Treat Options Oncol 9:215–233
Sangisetty SL, Miner TJ (2012) Malignant ascites: a review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg 4:87–95
Hodge C, Badgwell BD (2019) Palliation of malignant ascites. J Surg Oncol 120:67–73
Saif MW, Siddiqui IA, Sohail MA (2009) Management of ascites due to gastrointestinal malignancy. Ann Saudi Med 29:369–377
Ayantunde AA, Parsons SL (2007) Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol 18:945–949
Jehn CF, Küpferling S, Oskay-Özcelik G et al (2015) A survey of treatment approaches of malignant ascites in Germany and Austria. Support Care Cancer 23:2073–2078
Hisanaga T, Shinjo T, Imai K et al (2019) Clinical guidelines for management of gastrointestinal symptoms in cancer patients: the Japanese Society of Palliative Medicine recommendations. J Palliat Med 22:986–997
Maeda I, Morita T, Yamaguchi T et al (2016) Effect of continuous deep sedation on survival in patients with advanced cancer (J-Proval): a propensity score-weighted analysis of a prospective cohort study. Lancet Oncol 17:115–122
Materstvedt LJ, Bosshard G (2009) Deep and continuous palliative sedation (terminal sedation): clinical-ethical and philosophical aspects. Lancet Oncol 10:622–627
Papavasiliou EE, Payne S, Brearley S et al (2014) Current debates on end-of-life sedation: an international expert elicitation study. Support Care Cancer 22:2141–2149
Hiratsuka Y, Yamaguchi T, Maeda I et al (2020) The Functional Palliative Prognostic Index: a scoring system for functional prognostication of patients with advanced cancer. Support Care Cancer 28:6067–6074
Wimberger P, Gilet H, Gonschior AK et al (2012) Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol 23:1979–1985
Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10:37–48
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34:3661–3679
Desai RJ, Franklin JM (2019) Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 367:l5657
Hernán MA RJ (2020) Causal inference: what if. CRC: Boca Raton: Chapman & Hall
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30:377–399
Rubin DB (2004) Multiple imputation for nonresponse in surveys. John Wiley & Sons
Ito T, Yokomichi N, Ishiki H et al (2021) Optimal paracentesis volume for terminally ill cancer patients with ascites. J Pain Symptom Manage 62:968–977
Preston NJ, Fayers P, Walters SJ et al (2013) Recommendations for managing missing data, attrition and response shift in palliative and end-of-life care research: part of the MORECare research method guidance on statistical issues. Palliat Med 27:899–907
Hui D, Glitza I, Chisholm G et al (2013) Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer 119:1098–1105
Acknowledgements
We highly appreciate the participation of the patients and their families, and the cooperation of members of the East Asian Collaborative Cross-cultural Study to Elucidate the Dying Process (EASED) study group.
Funding
This work was supported by Grant-in-Aid from the Japanese Hospice Palliative Care Foundation and JSPS KAKENHI (Grant Number JP20K16567).
Author information
Authors and Affiliations
Contributions
KM, HI, TI, NY, TK, and MM made substantial contributions to the conception and design of the study, acquisition of data, and data analysis. KM and HI drafted the manuscript and approved the submitted version. HI made substantial contributions to the study design and revision of the manuscript. NY and TY accessed and verified the underlying study data and contributed to data analysis and interpretation. TI, HT, KA, SH, TY, YM, and TY contributed to the conception and study design, data collection and assembly, data interpretation, writing of the manuscript, and critical revision of the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The EASED study was performed in accordance with the ethical standards of the Helsinki declaration and the ethical guidelines for epidemiological research presented by the Ministry of Health, Labour and Welfare in Japan. The study protocol was reviewed and approved by the local institutional review boards of all participating institutions.
Consent to participate
Japanese law does not require individual informed consent from participants in a non-invasive observational trial. Therefore, we used an opt-out method rather than acquiring written or oral informed consent.
Consent for publication
Not applicable.
Conflict of interest
Dr. Ishiki reports personal fees from Mundipharma, Morinaga Clinico, Merck Serono, and Guerbet Japan and non-financial support from Shionogi outside of the submitted work; Dr. Masuda reports personal fees from Chugai and AstraZeneca outside of the submitted work. All other authors state that they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Masuda, K., Ishiki, H., Yokomichi, N. et al. Effect of paracentesis on the survival of patients with terminal cancer and ascites: a propensity score–weighted analysis of the East Asian Collaborative Cross-cultural Study to Elucidate the Dying Process. Support Care Cancer 30, 6233–6241 (2022). https://doi.org/10.1007/s00520-022-07057-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07057-8