Abstract
Purpose
This study was conducted to describe the portfolio of symptom science research conducted through the community oncology network supported by the US National Cancer Institute during the 12-year period 2008 to 2019.
Methods
The National Cancer Institute conducted a retrospective review of the National Cancer Institute database to identify pediatric and adult symptom management studies that were opened between 2008 and 2019 in the community oncology network and to determine types of studies, accrual patterns, completed studies, and number of publications reporting clinical trial results.
Results
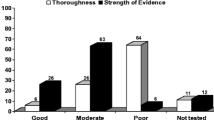
The NCI community oncology network conducted 109 symptom studies between 2008 and 2019. The majority of these studies were phase II and III clinical trials. Neurotoxicities were the most frequently occurring symptom studied, with the majority of those focused on neurocognitive impairments. Gastrointestinal symptoms, pain, and fatigue were the next most frequently studied. A variety of interventions were utilized including pharmacologic, behavioral, complementary and alternative medicines, and radiation therapy. Accrual to symptom studies ranged from a low of 896 participants in 2008 to a high of 3468 participants in 2012. The number of open studies ranged from 8 in 2008 to 35 in 2012.
Conclusions
Examining the symptom science portfolio of the NCI community oncology network has identified research gaps and has highlighted the need to focus on a mechanistic understanding of symptoms and phenotyping of patients experiencing cancer and treatment-related symptoms. Subsequently, targeted interventions can be developed to prevent or treat these symptoms.



Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Cancer Statistics. https://www.cancer.gov/about-cancer/understandingstatistic. Accessed 2 April 2021
Cleeland CS, Zhao F, Chang VT, Sloan JA, O’Mara AM, Gilman PB, Weiss M, Mendoza TR, Lee JW, Fisch MJ (2013) The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer 119(24):4333–40. https://doi.org/10.1002/cncr.28376
Reilly CM, Bruner DW, Mitchell SA (2013) A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer 21(6):1525–50. https://doi.org/10.1007/s00520-012-1688-0
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ (2016) Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 51(6):1070-1090.e9. https://doi.org/10.1016/j.jpainsymman.2015.12.340
Wang XS, Zhao F, Fisch MJ et al (2014) Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 120(3):425–32. https://doi.org/10.1002/cncr.28434
Fleming L, Randell K, Stewart E et al (2019) Insomnia in breast cancer: a prospective observational study. Sleep 42(3):zsy245. https://doi.org/10.1093/sleep/zsy245
Yates P, Miaskowski C, Cataldo JK et al (2015) Differences in composition of symptom clusters between older and younger oncology patients. J Pain Symptom Manage 49(6):1025–34. https://doi.org/10.1016/j.jpainsymman.2014.11.296
ASCO Clinical Practice Guidelines. https://ascopubs.org/jco/special/guidelines. Accessed 31 December 2020
ONS Symptom Interventions and Guidelines. https://www.ons.org/acq-search?search=clinical%20practice%20&source=Symptom%20Interventions. Accessed 31 December 2020
NCCN Guidelines for Supportive Care. https://www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed 31 December 2020
Paice JA, Portenoy R, Lacchetti C et al (2016) Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 34(27):3325–45. https://doi.org/10.1200/JCO.2016.68.5206
Hershman DL, Lacchetti C, Dworkin R et al (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32(18):1941–67. https://doi.org/10.1200/JCO.2013.54.0914
Description of NCORP. https://ncorp.cancer.gov/about/. Accessed 20 December 2021
Dodd M, Janson S, Facione N et al (2001) Advancing the science of symptom management. J Adv Nurs 33(5):668–76. https://doi.org/10.1046/j.1365-2648.2001.01697.x
Definition of cancer control. https://cancercontrol.cancer.gov/od/about.html. Accessed 31 December 2020
Definition of cancer prevention. https://www.cancer.gov/about-cancer/causes-prevention. Accessed 31 December 2020
Definition of cancer screening. https://www.cancer.gov/about-cancer/screening/hp-screening-overview-pdq. Accessed 31 December 2020
Definition of cancer care delivery. https://healthcaredelivery.cancer.gov/. Accessed 31 December 2020
Navari RM, Qin R, Ruddy KJ et al (2016) Olanzapine for the prevention off chemotherapy-induced nausea and vomiting. NEJM 375(2):134–42. https://doi.org/10.1056/NEJMoa1515725
Brown PD, Gondi V, Pugh S et al (2020) Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology cc001. J Clin Oncol 38(10):1019–1029. https://doi.org/10.1200/jco.19.02767
Lavoie Smith EM, Pang H, Cirrincione C et al (2013) Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 309(13):1359–67. https://doi.org/10.1001/jama.2013.2813
Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Palesh OG, Chandwani K, Reddy PS, Melnik MK, Heckler C, Morrow GR (2013) Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol 31(26):3233–41. https://doi.org/10.1200/JCO.2012.43.7707
Kleckner IR, Kamen C, Gewandter JS et al (2018) Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer 26(4):1019–1028. https://doi.org/10.1007/s00520-017-4013-0
Barton D et al (2013) Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 105(16):1230–8
Hershman DL, Unger JM, Greenlee H et al (2018) Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early stage breast cancer: a randomized clinical trial. JAMA 320(2):167–176. https://doi.org/10.1001/jama.2018.8907
Ryan JL, Heckler CE, Roscoe JA, Dakhil SR, Kirshner J, Flynn PJ, Hickok JT, Morrow GR (2012) Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer 20(7):1479–89. https://doi.org/10.1007/s00520-011-1236-3
Clinical characterization of cancer therapy-induced adverse sequelae and mechanism-based interventional strategies. https://grants.nih.gov/grants/guide/pa-files/par-19-325.html Accessed 31 December 2020
Institute of Medicine and National Research Council. 2001. Improving palliative care for cancer: summary and recommendations. The National Academies Press. Washington, DC. https://doi.org/10.17226/10147
Bruner DW, Movsas B, Konski A et al (2004) Outcomes research in cancer clinical trial cooperative groups: the RTOG model. Qual Life Res 13(6):1025–41. https://doi.org/10.1023/B:QURE.0000031335.02254.3b
Bruner DW (2007) Outcomes research in cancer symptom management trials: the Radiation Therapy Oncology Group (RTOG) conceptual model. J Natl Cancer Inst Monogr 37:12–15. https://doi.org/10.1093/jncimonographs/lgm004
Bruner DW, Bryan CJ, Aaronson N (2007) National Cancer Institute issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol 25(32):5051–7. https://doi.org/10.1200/JCO.2007.11.3324
Cleeland CS, Fisch MF, and Dunn AJ (eds) (2010) Cancer symptom science: measurement, mechanisms, and management. Cambridge University Press, New York
Miaskowski C, Barsevick A, Berger A et al (2017) Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst 109(4):djw253. https://doi.org/10.1093/jnci/djw253
Dantzer R, Meagher MW, Cleeland CS (2012) Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol 9(7):414–26. https://doi.org/10.1038/nrclinonc.2012.88
Filler K, Saligan LN (2016) Defining cancer-related fatigue for biomarker discovery. Support Care Cancer 24(1):5–7. https://doi.org/10.1007/s00520-015-2965-5
Dorsey SG, Kleckner IR, Barton D et al (2019) The National Cancer Institute clinical trials planning meeting for prevention and treatment of chemotherapy-induced peripheral neuropathy. J Natl Cancer Inst 111(6):531–537. https://doi.org/10.1093/jnci/djz011
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The data analysis was solicited by Worta McCaskill-Stevens (Director, NCI Community Oncology Research Program). Material preparation, data collection, and analysis were performed by Diane St. Germain and Ann O’Mara. The first draft of the manuscript was written by Ann O’Mara and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key message
This article describes symptom science conducted through the community oncology network supported by the US National Cancer Institute over the recent 12-year period. One hundred and nine studies were identified, the majority of which were phase II and III clinical trials testing an agent. The most frequent symptom studied was neurotoxicity, with most of those trials focused on neurocognition.
Rights and permissions
About this article
Cite this article
Germain, D.S., Stevens, W.M. & O’Mara, A. Symptom science research conducted in community programs funded by the US National Cancer Institute: a 12-year review, 2008 to 2019. Support Care Cancer 30, 4739–4746 (2022). https://doi.org/10.1007/s00520-022-06875-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06875-0