Abstract
Background
Despite the frequency of vasomotor symptoms (VMS) in patients with early breast cancer (EBC), their optimal management remains unknown. A patient survey was performed to determine perspectives on this important clinical challenge.
Methods
Patients with EBC experiencing VMS participated in an anonymous survey. Patients reported on the frequency and severity of VMS using the validated Hot Flush Rating Scale (HFRS) and ranked their most bothersome symptoms. Respondents were also asked to determine endpoints that defined effective treatment of VMS and report on the effectiveness of previously tried interventions.
Results
Responses were received from 373 patients, median age 56 years (range 23–83), who experienced an average of 5.0 hot flashes per day (SD 6.57). Patients reported the most bothersome symptoms to be feeling hot/sweating (155/316, 49%) and sleeping difficulties (86/316, 27%). Fifty-five percent (201/365) of patients would consider a treatment to be effective if it reduced night-time awakenings. While 68% of respondents were interested in trying interventions from their healthcare team to manage VMS, only 18% actually did so. Of the 137 patients who had tried an intervention for VMS, pharmacological treatments, exercise, and relaxation strategies were more likely to be effective, while therapies such as melatonin and black cohosh were deemed less effective.
Conclusion
VMS are a common and bothersome problem for EBC patients, with a minority receiving interventions to manage these symptoms. Further research is needed to identify patient-centered strategies for managing these distressing symptoms.
Similar content being viewed by others
Introduction
Vasomotor symptoms (VMS) are a common consequence of systemic therapies for breast cancer. These treatments, including endocrine therapies, ovarian function suppression, and chemotherapy, suppress endogenous estrogen levels through various mechanisms, ultimately leading to imbalances in serotonin concentrations, and disruptions in thermoregulation [1]. Although hot flashes and sweats are the most common symptoms, patients can experience many others including disturbances in sleep, mood, and cognition [2, 3]. Breast cancer treatments can cause VMS in approximately 30% of postmenopausal women [4, 5] and 95% of premenopausal women [6] with early breast cancer (EBC). Moreover, for post-menopausal women experiencing VMS prior to treatment initiation, intensity of symptoms may be exacerbated following the introduction of endocrine therapies [7]. In addition to their negative impact on quality of life, unmanaged VMS are the most common reason for discontinuation of potentially curative treatment in 25–60% of EBC patients [8,9,10,11,12]. Treatment discontinuation is associated with an increased risk of breast cancer recurrence and reduced survival [8]. As adjuvant endocrine therapy may be prescribed for up to 10 years following a breast cancer diagnosis [13, 14], appropriate strategies for the identification and management of patients experiencing bothersome VMS are essential to improve patient quality of life and breast cancer outcomes.
There are many non-estrogen-based interventions available to manage VMS, including lifestyle modifications (e.g., dressing in layers), complementary and alternative medicine (CAM) therapies (e.g., black cohosh [15], melatonin [16], exercise therapy, acupuncture [17]), prescription medications (e.g., venlafaxine [18], gabapentin [19, 20]), and adjustment of anticancer therapy (e.g., dose reduction or alternate agents). However, a recent systematic review and meta-analysis of pharmacological and CAM interventions found no single optimal treatment for VMS management in breast cancer patients [21]. This was because often the data was of poor quality, with small sample sizes, heterogeneous patient populations, limited integration of patient derived endpoints, and infrequent comparisons of different effective interventions [21]. Furthermore, despite numerous treatments and recommendations from breast cancer guideline groups [22, 23], a recent survey of oncology healthcare professionals (HCP) found that many providers lacked confidence in managing VMS and were uncertain about the efficacy of treatments [19].
In the current study, we surveyed patients with EBC to identify the most bothersome symptoms associated with VMS, to determine a definition of optimal control of VMS, and to obtain patient perspectives on the effectiveness of previously tried management strategies. The results of this survey will be used to aid in the design of future prospective interventional trials for VMS management.
Materials and methods
Study population
Women aged 18 years of age and older with EBC (stage I–III) and VMS were included in this study. Patients were recruited from two cancer centers located in Ottawa, Ontario, and London, Ontario.
Study objectives
The major objectives of this study were to (1) identify which VMS are most bothersome for patients, (2) identify a patient-derived definition of optimal control of VMS, and (3) determine the perceived efficacy of previous interventions for VMS based on these patient derived endpoints.
Survey development
This survey was developed by physicians, nurses, and researchers with expertise in both breast cancer management and survey development. The first section of the survey consisted of one mandatory question to determine eligibility, and one question obtaining demographic data. Once these questions were answered, all subsequent questions were optional, and as such, the response rate varies between questions. Section two included questions related to breast cancer treatment history, menopausal status, and how often patients were asked about VMS in the clinic.
Section three asked patients to rate their hot flash frequency and severity using the Hot Flush Rating Scale (HFRS) [24] and rank their most bothersome VMS. The HFRS is a validated tool which evaluates (1) hot flash and night sweat frequency and (2) hot flash severity by means of a Hot Flush Night Sweats (HFNS) problem rating score. The HFNS problem rating score evaluates the degree to which VMS are problematic, distressing, or disruptive to daily life, with each of the 3 factors evaluated on a 10-point scale where 0 means not a problem, and 10 represents a significant problem. Questions related to the ability to cope and control hot flashes were also included; however, these factors were found to be less reliable in the original HFRS validation study and are not included in the HFNS problem rating score [24].
In section four, patients were asked to define what a meaningful improvement in VMS would be for them, to report treatments or interventions that they had received for VMS, and to comment on their perceived effectiveness. “Treatments” for VMS were defined as drug and/or complementary therapies and did not include modifications to their endocrine therapy. In addition, an exploratory follow-up question was added as a protocol amendment in November 2020, to determine whether patients experienced changes in VMS following alterations in their anti-cancer therapy. The final section of the survey asked patients to identify VMS interventions that they would be interested in trying.
Survey implementation
Eligible patients were approached by a member of their breast cancer care team at their routine clinical visit. As the survey was conducted during the COVID-19 pandemic, most patients were approached during virtual visits. Patients discharged from the clinic who were being followed by oncology nurse practitioners or their family doctor were also invited to participate after consulting a database of breast cancer patients maintained by the Ottawa Hospital’s Wellness Beyond Cancer Program. Patients who agreed to participate in the survey were then approached by the study research coordinator, who provided patients with a link to the electronic survey on Microsoft Forms, or mailed a paper version of the survey if they preferred. All patients that completed the survey by email received an automatic reminder 2 weeks after consenting to participate. The survey was approved by the Ontario Cancer Research Ethics Board (OCREB).
Sample size
The primary objective of this study was to get accurate estimates of the impact of VMS on patients. Thus, the sample size was derived to ensure that estimates were sufficiently precise to make reasonable inferences on patient reported results. A 95% confidence interval for a dichotomous outcome has maximum width when the estimated response rate is 0.5. Thus, to ensure the width of a 95% CI is < 0.10 (i.e., the 95% CI would range from 0.45 to 0.55 when the response rate is 0.50), a sample size of 400 EBC patients from two cancer centers in Ontario (Ottawa, Ontario and London, Ontario) was targeted.
Data analysis
Patient characteristics and outcomes were summarized using descriptive statistics. The primary goals of this survey were to describe patient perspectives and acquire estimates, and thus, no statistical testing was performed. Two-sided, 95% confidence intervals were constructed for selected results to better interpret estimates.
Results
The survey was conducted between June 5, 2020 and March 5, 2021. Study initiation was delayed due to the onset of the COVID-19 pandemic in Canada, and accrual at all sites was impacted by the pandemic throughout the study. Sixteen patients were accrued from the London Regional Cancer Centre with the remaining 357 patients from The Ottawa Hospital Cancer Centre. Survey completion rates at the Ottawa Hospital were 80% (n = 357/448). In total, 383 patients completed the survey, with 373 patients fulfilling eligibility criteria. The 10 patients who were ineligible were excluded as they were not experiencing VMS at the time the survey was conducted.
The median age of the eligible patients was 56 years (range of 23–83) (Table 1) and the majority of respondents reported being post-menopausal at the time of survey completion (207/373, 55.4%) (Table 1). Forty-six percent (110/239) of patients reported experiencing VMS before their cancer diagnosis and 45.6% (109/239) experienced them only after their diagnosis. The most common cancer therapies patients had received were endocrine therapy (329/373, 88.2%), chemotherapy (211/373, 56.6%), and ovarian function suppression (73/373, 19.6%). Many patients (215/373, 57.6%) reported that they were routinely asked about VMS in clinic visits.
Subjective experience of VMS
The mean number of hot flashes per day was 5.02 (SD 6.57) and the average number of night sweats per night was 2.19 (SD 2.41) (Table 2). Patients were asked to respond to questions pertaining to their subjective experience of VMS utilizing the HFNS problem rating score. When asked to respond to the question “to what extent do you regard your hot flashes/night sweats as a problem?” (0 not a problem and 10 very much a problem), the mean score was 5.10 (SD 2.79) (Table 2). When asked about distress caused by VMS, the mean score was 4.28 (SD 2.88), and the mean score for VMS interference with daily life was 3.37 (SD 2.70). The mean cumulative HFNS problem score, incorporating “problem,” “distress,” and “interference” questions, was 4.24 (SD 2.57). Although patients reported some ability to cope with VMS, with a mean score of 6.96 (SD 2.44), patients did not feel they had much control over their symptoms, with a mean score of 3.26 (SD 3.23) (Table 2).
Patients were asked to rank their 1st, 2nd, and 3rd most bothersome symptoms. The 1st most common bothersome symptoms were “feeling extremely hot and sweaty” (155/316, 49.1%), “difficulties sleeping” (86/316, 27.2%), and “redness of the face/chest” (11/316, 3.5%) (Table 3). The 2nd most common bothersome symptoms were feeling extremely hot and sweaty (84/307, 27.4%), “difficulties sleeping” (62/307, 20.2%), and “feeling chilly/clammy” (32/307, 10.4%). Finally, the 3rd most common bothersome symptoms were “feeling chills/clammy” (47/303, 15.6%), “difficulties sleeping” (37/303, 12.2%), and irritability (23/303, 7.6%). Of note, although all 373 patients responded to the initial question of this set, 57 patients (18%) were excluded as respondents provided either more than 3 symptoms of concern, or 3 symptoms with no ranking provided. When asked whether hot flash frequency or severity were more bothersome for patients, the majority (171/373, 46%) indicated that severity and frequency were equally bothersome (Table 3).
Interventions for VMS
Eighty percent (300/373) of patients reported that they had not received any formal treatments or health-care provider recommendations for their VMS (Table 4). Of the patients who had received a prescribed drug intervention for VMS, the most common were anti-depressants (39/68, 57.4%), gabapentin (8/68, 11.8%), and clonidine (6/68, 8.8%). The most common over-the-counter supplements utilized were melatonin (11/68, 16.1%), black cohosh (10/68, 14.7%), and evening primrose oil (5/68, 7.4%). Twenty-five patients reported receiving a recommendation for other complementary or alternative therapies, with the most common responses being exercise therapy or yoga (11/25, 44.0%), acupuncture (6/25, 24.0%), and relaxation therapy (6/25, 24.0%). Only a minority of patients (22/371, 5.9%) reported being referred to a specialized menopause clinic.
The majority of patients (286/373, 76.7%) indicated that “no changes” were made to their anti-cancer therapies due to bothersome VMS (Table 4). For those who reported that changes were made, the most common intervention was a change in the treatment dose (38/373, 10.2%). Six months into the survey an exploratory question was added asking patients if changes to systemic therapy improved their VMS (Table 4).
Effective control of VMS
Patients were asked how they would define effective control of VMS. They were presented with five options, with the opportunity to select multiple options and/or an “other” response. The most common response was that an effective treatment would improve night-time awakenings (201/365, 55.0%), with other common choices including “decreased frequency of hot flashes” (161/365, 44.1%) and decreased severity of hot flashes (151/365, 41.3%) (Table 5).
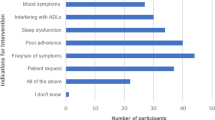
Based on their definition of effective control of VMS, patients were then asked to indicate which previously tried drug and/or complementary interventions adequately controlled their VMS. Importantly, a total of 137 patients commented on specific treatments that they found effective. Of those who reported trying an antidepressant, 60% (40/66) reported that this intervention adequately controlled their symptoms (Fig. 1). Other common interventions that the majority of patients found to be effective included exercise (39/49, 79.5%), gabapentin (10/15, 66.7%), relaxation therapy (10/15, 66.7%), and hormone replacement therapy (10/15, 66.7%). The majority of patients referred to a dedicated menopause clinic for specialist care found it beneficial (10/12, 83.3%). Common interventions felt by the majority of patients to be ineffective included melatonin (18/30, 60%), black cohosh (12/20, 60%), and evening primrose (12/13, 92.3%).
When asked about their interest in pursuing an intervention for VMS, 32% (116/361) of patients declined, indicating that their symptoms were managed with lifestyle modifications alone. Of the 68% of patients interested in an intervention, 3% (12/361) would consider a prescription medication, with greater preference for a “vitamin/supplement” (97/361, 26.9%) or “complementary therapy” (38/361, 10.5%), while 21% (76/361) would consider “any option if it improves symptoms.”
Discussion
Vasomotor symptoms are a common and bothersome problem following treatment for EBC and their management remains a significant unmet clinical need for many patients. While natural menopause contributes to VMS in post-menopausal EBC patients, research has established that breast cancer treatments, including endocrine therapies, can exacerbate these symptoms [7], with negative impacts on QOL [2, 3], compliance with cancer treatments [8,9,10,11], and ultimately breast cancer outcomes [12]. Our study has identified that in a large, real-world patient population, the extent to which VMS cause problematic and distressing symptoms varies between patients. This highlights the need for patient-centered strategies to identify and offer treatment a priori to those patients at higher risk of developing debilitating symptoms that may impact quality of life and compliance with adjuvant treatments. This would also help clinicians develop personalized treatment strategies for patients wanting to receive interventions for VMS.
Patients reported being most bothered by sleeping difficulties and sweats associated with VMS, and most would consider a treatment to be effective if it reduced night-time awakenings. This is an important finding, as sleep-related endpoints have only been evaluated in a minority of trials evaluating interventions for VMS [21].
With respect to the effectiveness of prior interventions, patients were more likely to find anti-depressants, exercise, gabapentin, relaxation therapy, and referral to a dedicated menopause clinic to be effective, while melatonin, black cohosh, and evening primrose oil were more likely to be deemed ineffective in managing symptoms (Fig. 1) (Online Resource 1). Although hormone replacement therapy was listed as more likely to be an effective treatment, this is generally contra-indicated in patients with hormone positive breast cancer due to the role of estrogen in tumorigenesis. Overall, given the small numbers of patients who received treatments for VMS, it is difficult to draw definitive conclusions on the efficacy of specific interventions from our study.
The majority of patients were interested in trying interventions to improve VMS although there was a preference for non-prescription treatment options. Thirty-two percent of patients did report that lifestyle modifications alone adequately managed their symptoms. This is important when compared to our recent HCP survey where physicians reported greater preference and experience in recommending prescription medications, particularly anti-depressants [25]. This highlights the need for greater education for HCP on patient-centered treatment preferences, and possibly the initial use of non-prescription treatment options for VMS if the patient so wishes.
There are several limitations to this study. Our survey focused on patients receiving treatment for EBC and actively experiencing VMS. We did not survey patients prior to commencing breast cancer treatment, nor did we include patients without VMS. Therefore, we cannot comment on treatment emergent VMS, particularly in patients already experiencing natural menopause. However, this question has been addressed by previous studies, where breast cancer therapies have been found to exacerbate natural menopause [7]. The cross-sectional nature increases the risk of recall bias, and patients may not have accurately remembered their previous treatments and their effectiveness. Previous treatments for VMS were not further corroborated in the chart, leading to the potential for inaccurate reporting. Moreover, the responses to similar questions varied within the survey. For example, while 69 patients indicated that their health care provider recommended or prescribed an intervention, 137 patients commented on the effectiveness of previously tried therapies. These discrepancies may be secondary to patients taking or being prescribed these interventions for reasons other than their hot flashes, or trying interventions outside of HCP recommendations. As many of the survey questions were optional, the number of respondents varied depending on the question. This may have impacted study power to answer certain questions, and we will consider this limitation in the design of future surveys. Furthermore, in the paper version of the survey some of the questions were answered incorrectly, requiring the exclusion of data. As this occurred exclusively in patients who completed the paper version of the survey, transitioning to fully electronic surveys in the future will help to address this issue and ensure that questions are easier to understand and complete for participants. Finally, this study was conducted during the COVID-19 pandemic, which led to delayed start times, slower than anticipated patient accrual, and decreased involvement from other participating sites.
Clearly more studies are needed. Improved patient-centered strategies are required to identify patients at risk of developing significant VMS a priori, and personalized treatment algorithms are needed to guide management of patients. Our future work will include the integration of machine learning in a prospective study, as this type of approach is ideally suited to the analysis of data with multiple variables as is the case with VMS [26]. This work, which will collect additional data on menopausal symptoms experienced prior to systemic therapy initiation, will provide additional insights into the interaction between “natural” menopause and treatment-induced menopausal symptoms.
Conclusions
Our data shows that the extent to which patients experience problematic VMS varies widely among the EBC population, yet only a minority of patients are receiving treatment for this problem. An effective treatment for patients would be one that reduces nocturnal awakenings and improves sleep. A variety of interventions are available for the treatment of VMS, but more research is required to identify patients at greatest risk for VMS and to provide personalized, patient-centered management strategies.
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on request with approval of the Ontario Cancer Research Ethics Board.
References
Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D (2002) Hot flushes. Lancet 360(9348):1851–1861. https://doi.org/10.1016/s0140-6736(02)11774-0
Utian WH (2005) Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 3:47. https://doi.org/10.1186/1477-7525-3-47
Woods NF, Mitchell ES (2005) Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med 118(Suppl 12B):14–24. https://doi.org/10.1016/j.amjmed.2005.09.031
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, ATAC Trialists’ Group (2005) Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62. https://doi.org/10.1016/S0140-6736(04)17666-6
Regan MM, Price KN, Giobbie-Hurder A, Thürlimann B, Gelber RD, Group IBCSGaB-C (2011) Interpreting Breast International Group (BIG) 1–98: a randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive, early breast cancer. Breast Cancer Res 13(3):209. https://doi.org/10.1186/bcr2837
Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, Gomez HL, Tondini C, Burstein HJ, Perez EA, Ciruelos E, Stearns V, Bonnefoi HR, Martino S, Geyer CE Jr, Pinotti G, Puglisi F, Crivellari D, Ruhstaller T, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Bernhard J, Luo W, Ribi K, Viale G, Coates AS, Gelber RD, Goldhirsch A, Francis PA, TEXT and SOFT Investigators, International Breast Cancer Study Group (2014) Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371(2):107–118. https://doi.org/10.1056/NEJMoa1404037
Ganz PA, Petersen L, Bower JE, Crespi CM (2016) Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body study. J Clin Oncol 34(8):816–824. https://doi.org/10.1200/JCO.2015.64.3866
Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, Gelber RD, Goldhirsch A, Smith I, Rabaglio M, Forbes JF, Neven P, Láng I, Colleoni M, Thürlimann B (2016) Treatment adherence and its impact on disease-free survival in the Breast International Group 1–98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol 34(21):2452–2459. https://doi.org/10.1200/JCO.2015.63.8619
Yanez B, Gray RJ, Sparano JA, Carlos RC, Sadigh G, Garcia SF, Gareen IF, Whelan TJ, Sledge GW, Cella D, Wagner LI (2021) Association of modifiable risk factors with early discontinuation of adjuvant endocrine therapy: a post hoc analysis of a randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2021.1693
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128. https://doi.org/10.1200/JCO.2009.25.9655
McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, Fahey TP (2008) Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 99(11):1763–1768. https://doi.org/10.1038/sj.bjc.6604758
Yood MU, Owusu C, Buist DS, Geiger AM, Field TS, Thwin SS, Lash TL, Prout MN, Wei F, Quinn VP, Frost FJ, Silliman RA (2008) Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg 206(1):66–75. https://doi.org/10.1016/j.jamcollsurg.2007.07.015
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Müller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrútia G, Valentini M, Wang Y, Peto R, Group ATLASAC (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805–816. https://doi.org/10.1016/S0140-6736(12)61963-1
Goss PE (2007) Letrozole in the extended adjuvant setting: MA.17. Breast Cancer Res Treat 105(Suppl 1):45–53. https://doi.org/10.1007/s10549-007-9698-1
Hernández Muñoz G, Pluchino S (2003) Cimicifuga racemosa for the treatment of hot flushes in women surviving breast cancer. Maturitas 44(Suppl 1):S59-65. https://doi.org/10.1016/s0378-5122(02)00349-3
Chen WY, Giobbie-Hurder A, Gantman K, Savoie J, Scheib R, Parker LM, Schernhammer ES (2014) A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat 145(2):381–388. https://doi.org/10.1007/s10549-014-2944-4
Lesi G, Razzini G, Musti MA, Stivanello E, Petrucci C, Benedetti B, Rondini E, Ligabue MB, Scaltriti L, Botti A, Artioli F, Mancuso P, Cardini F, Pandolfi P (2016) Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: a prospective multicenter randomized controlled trial (AcCliMaT). J Clin Oncol 34(15):1795–1802. https://doi.org/10.1200/JCO.2015.63.2893
Boekhout AH, Vincent AD, Dalesio OB, van den Bosch J, Foekema-Töns JH, Adriaansz S, Sprangers S, Nuijen B, Beijnen JH, Schellens JH (2011) Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 29(29):3862–3868. https://doi.org/10.1200/JCO.2010.33.1298
Loprinzi CL, Kugler JW, Barton DL, Dueck AC, Tschetter LK, Nelimark RA, Balcueva EP, Burger KN, Novotny PJ, Carlson MD, Duane SF, Corso SW, Johnson DB, Jaslowski AJ (2007) Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 25(3):308–312. https://doi.org/10.1200/JCO.2006.07.5390
Bordeleau L, Pritchard KI, Loprinzi CL, Ennis M, Jugovic O, Warr D, Haq R, Goodwin PJ (2010) Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol 28(35):5147–5152. https://doi.org/10.1200/JCO.2010.29.9230
Hutton B, Hersi M, Cheng W, Pratt M, Barbeau P, Mazzarello S, Ahmadzai N, Skidmore B, Morgan SC, Bordeleau L, Ginex PK, Sadeghirad B, Morgan RL, Cole KM, Clemons M (2020) Comparing interventions for management of hot flashes in patients with breast and prostate cancer: a systematic review with meta-analyses. Oncol Nurs Forum 47(4):E86–E106. https://doi.org/10.1188/20.ONF.E86-E106
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA (2016) American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin 66(1):43–73. https://doi.org/10.3322/caac.21319
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org (2019) Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 30(8):1194–1220. https://doi.org/10.1093/annonc/mdz173
Hunter MS, Liao KL (1995) A psychological analysis of menopausal hot flushes. Br J Clin Psychol 34(4):589–599. https://doi.org/10.1111/j.2044-8260.1995.tb01493.x
Cole KM, Clemons M, Alzahrani M, Larocque G, MacDonald F, Vandermeer L, Hutton B, Piper A, Pond G, McGee S (2021) Developing patient-centred strategies to optimize the management of vasomotor symptoms in breast cancer patients: a survey of health care providers. Breast Cancer Res Treat 188(2):343–350. https://doi.org/10.1007/s10549-021-06186-8
Cole K, Clemons M, El Emam K, Larocque G, MacDonald F, Vandermeer L, Hutton B, Piper A, Pong G, McGee S (2021) Integrating machine learning models into the management of vasomotor symptoms in breast cancer patients [abstract]. Abstracts of the 2021 Canadian Association of Medical Oncologists annual meeting. Curr. Oncol 28(3), 2199-2226. https://doi.org/10.3390/curroncol28030204
Funding
This work was supported by the Rethinking Clinical Trials (REaCT) program at the Ottawa Hospital, which is supported by The Ottawa Hospital Foundation and its donors.
Author information
Authors and Affiliations
Contributions
KC, SMG, MC, MA, LV, FM, AP, and GL designed the survey and prepared the protocol. ML collected the data and coordinated the study. KC did the statistical analysis and wrote the manuscript. All authors had full access to the data and take responsibility for the integrity of the data and accuracy of the data analysis. All authors were involved in the critical review of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ontario Cancer Research Ethics Board (OCREB).
Consent
Informed consent was obtained from all individual participants included in the study, including consent to publish study findings.
Competing interests
Mark Clemons reports honoraria from Pfizer, outside submitted work. Brian Hutton reports consulting fees from Cornerstone Research and Eversana, outside submitted work. Gregory Pond reports receipt of honorarium from Astra-Zeneca, Merck, Takeda, all outside the submitted work, and has a family member who is an employee of Roche and owns stock in Roche. Khaled El Emam holds equity in Replica Analytics Ltd., a software company spin-off from the University of Ottawa that develops synthetic data generation software. Sharon McGee reports honoraria from Novartis, outside the submitted work. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cole, K.M., Clemons, M., Alzahrani, M. et al. Vasomotor symptoms in early breast cancer—a “real world” exploration of the patient experience. Support Care Cancer 30, 4437–4446 (2022). https://doi.org/10.1007/s00520-022-06848-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06848-3