Abstract
Context
Taste, smell, and mouthfeel disturbances are underrated and underreported, but important side effects of anti-cancer medication. These symptoms are associated with a lower quality of life (QoL). The prevalence and the impact of taste, smell, and mouthfeel disturbances on daily life in patients with a gastrointestinal stromal tumor (GIST) are largely unknown.
Objectives
This exploratory study assessed the prevalence and type of taste, smell, and mouthfeel disturbances and their impact on daily life and QoL in patients with a GIST treated with a tyrosine-kinase inhibitor (TKI).
Methods
Patients currently treated with TKIs for GIST completed a standardized questionnaire. The questionnaire addressed changes in taste, smell, and mouthfeel and, if changes occurred, impact on daily life and QoL. Statistics are descriptive.
Results
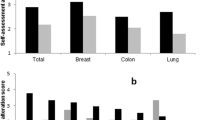
A total of 65 GIST patients on TKI treatment completed the questionnaire. Of these patients, 79%, 12%, and 9% currently used imatinib, sunitinib, and regorafenib respectively. Taste, smell, and mouthfeel disturbances were reported by 25 (38%), 15 (23%), and 36 (55%) patients respectively. Salty and sweet tastes were mostly affected, respectively in 14 and 13 patients. A dry mouth was experienced by 29 (45%) patients. Taste disturbances were more often reported to have impact on daily life and QoL (80% and 60%) than smell (47% and 31%) and mouthfeel disturbances (47% and 30%).
Conclusion
Taste, smell, and mouthfeel disturbances are frequent side effects of TKIs in GIST patients. Daily life and QoL are affected in a considerable number of those patients.
Trial registration
ClinicalTrials.gov Identifier: NL7827 (2019–06-25).


Similar content being viewed by others
References
Hovan AJ, Williams PM, Stevenson-Moore P et al (2010) A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer 18:1081–1087. https://doi.org/10.1007/s00520-010-0902-1
de Vries YC, Boesveldt S, Kelfkens CS et al (2018) Taste and smell perception and quality of life during and after systemic therapy for breast cancer. Breast Cancer Res Treat 170:27–34. https://doi.org/10.1007/s10549-018-4720-3
Boltong A, Aranda S, Keast R et al (2014) A prospective cohort study of the effects of adjuvant breast cancer chemotherapy on taste function, food liking, appetite and associated nutritional outcomes. PLoS ONE 9:e103512. https://doi.org/10.1371/journal.pone.0103512
Hutton JL, Baracos VE, Wismer WV (2007) Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J Pain Symptom Manage 33:156–165. https://doi.org/10.1016/j.jpainsymman.2006.07.017
Brisbois TD, de Kock IH, Watanabe SM, Baracos VE, Wismer WV (2011) Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manage 41:673–683. https://doi.org/10.1016/j.jpainsymman.2010.06.022
Lindley C, McCune JS, Thomason TE et al (1999) Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract 7:59–65. https://doi.org/10.1046/j.1523-5394.1999.07205.x
Zabernigg A, Gamper E-M, Giesinger JM et al (2010) Taste alterations in cancer patients receiving chemotherapy: a neglected side effect? Oncologist 15:913–920. https://doi.org/10.1634/theoncologist.2009-0333
Søreide K, Sandvik OM, Søreide JA et al (2016) Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol 40:39–46. https://doi.org/10.1016/j.canep.2015.10.031
Casali PG, Abecassis N, Aro HT et al (2018) Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Off J Eur Soc Med Oncol 29:iv68-78. https://doi.org/10.1093/annonc/mdy320
van der Werf A, Rovithi M, Langius JAE, de van der Schueren MAE, Verheul HMW (2017) Insight in taste alterations during treatment with protein kinase inhibitors. Eur J Cancer 86:125–34. https://doi.org/10.1016/j.ejca.2017.09.006
U.S. Department of Health and Human Services (2017) Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed September 4, 2021
Sodergren SC, White A, Efficace F et al (2014) Systematic review of the side effects associated with tyrosine kinase inhibitors used in the treatment of gastrointestinal stromal tumours on behalf of the EORTC Quality of Life Group. Crit Rev Oncol Hematol 91:35–46. https://doi.org/10.1016/j.critrevonc.2014.01.002
Demetri GD, von Mehren M, Blanke CD et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480. https://doi.org/10.1056/NEJMoa020461
Demetri GD, van Oosterom AT, Garrett CR et al (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368:1329–1338. https://doi.org/10.1016/S0140-6736(06)69446-4
Vigarios E, Epstein JB, Sibaud V (2017) Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer 25:1713–1739. https://doi.org/10.1007/s00520-017-3629-4
European Medicines Agency (2019) Stivarga: EPAR - Product Information. https://www.ema.europa.eu/en/documents/product-information/stivarga-epar-product-information_en.pdf. Accessed March 6, 2021
Joensuu H, Eriksson M, Sundby Hall K et al (2012) One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307:1265–1272. https://doi.org/10.1001/jama.2012.347
Farag S, van Coevorden F, Sneekes E et al (2017) Elderly patients with gastrointestinal stromal tumour (GIST) receive less treatment irrespective of performance score or comorbidity – a retrospective multicentre study in a large cohort of GIST patients. Eur J Cancer 86:318–325. https://doi.org/10.1016/j.ejca.2017.09.017
De Haan JJ, Moshage Y, Kluifhooft D, et al. (2021) Self-reported taste and smell alterations and the liking of oral nutritional supplements with sensory-adapted flavors in cancer patients receiving systemic antitumor treatment. Support Care Cancer. Epub ahead of print. https://doi.org/10.1007/s00520-021-06049-4
IJpma I, Timmermans ER, Renken RJ, Terhorst GJ, Reyners AKL (2017) Metallic taste in cancer patients treated with systemic therapy: a questionnaire-based study. Nutr Cancer 69:140–5. https://doi.org/10.1080/01635581.2017.1250922
Hochhaus A, Saussele S, Rosti G et al (2018) Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv261. https://doi.org/10.1093/annonc/mdy159
Morishima Y, Ogura M, Nishimura M et al (2004) Efficacy and safety of imatinib mesylate for patients in the first chronic phase of chronic myeloid leukemia: results of a Japanese phase II clinical study. Int J Hematol 80:261–266. https://doi.org/10.1532/ijh97.04074
van Oort S, Kramer E, de Groot J-W, Visser O (2018) Taste alterations and cancer treatment. Curr Opin Support Palliat Care 12:162–167. https://doi.org/10.1097/SPC.0000000000000346
Frowen J, Hughes R, Skeat J (2019) The prevalence of patient-reported dysphagia and oral complications in cancer patients. Support care cancer 28:1141–1150. https://doi.org/10.1007/s00520-019-04921-y
Davies AN, Broadley K, Beighton D (2001) Xerostomia in patients with advanced cancer. J Pain Symptom Manage 22:820–825. https://doi.org/10.1016/s0885-3924(01)00318-9
Barlow LA (2015) Progress and renewal in gustation: new insights into taste bud development. Development 142:3620–3629. https://doi.org/10.1242/dev.120394
Abdel-Aziz AK, Mantawy EM, Said RS, Helwa R (2016) The tyrosine kinase inhibitor, sunitinib malate, induces cognitive impairment in vivo via dysregulating VEGFR signaling, apoptotic and autophagic machineries. Exp Neurol 283:129–141. https://doi.org/10.1016/j.expneurol.2016.06.004
Croy I, Nordin S, Hummel T (2014) Olfactory disorders and quality of life-an updated review. Chem Senses 39:185–194. https://doi.org/10.1093/chemse/bjt072
Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME (2005) Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 27:659–668. https://doi.org/10.1002/hed.20221
Bromley SM (2000) Smell and taste disorders: a primary care approach. Am Fam Physician 61(427–436):438
Kabarriti R, Bontempo A, Romano M et al (2018) The impact of dietary regimen compliance on outcomes for HNSCC patients treated with radiation therapy. Support Care Cancer 26:3307–3313. https://doi.org/10.1007/s00520-018-4198-x
Mathey MF (2001) Assessing appetite in Dutch elderly with the Appetite, Hunger and Sensory Perception (AHSP) questionnaire. J Nutr Health Aging 5:22–28
Bauer J, Capra S, Ferguson M (2002) Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 56:779–785. https://doi.org/10.1038/sj.ejcn.1601412
Kano T, Kanda K (2013) Development and validation of a chemotherapy-induced taste alteration scale. Oncol Nurs Forum 40:E79-85. https://doi.org/10.1188/13.ONF.E79-E85
Funding
JVE was supported by the Junior Scientific Masterclass Groningen program of the Rijksuniversiteit Groningen.
Author information
Authors and Affiliations
Contributions
J.M. van Elst, A.K.L. Reyners, and J.J. de Haan contributed to the study conception and design. Material preparation and data collection were performed by J.M. van Elst and N.S. IJzerman. Analysis was done by J.M van Elst. The first draft of the manuscript was written by J.M. van Elst and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the UMCG, NKI, EMC (Ethics approval number: UMCG: METc 2019/360, NKI: IRBd19-258, EMC: MEC-2019–0669).
Consent to participate
Verbal informed consent was telephonically obtained prior to the interview.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Elst, J.M., IJzerman, N.S., Mathijssen, R.H.J. et al. Taste, smell and mouthfeel disturbances in patients with gastrointestinal stromal tumors treated with tyrosine-kinase inhibitors. Support Care Cancer 30, 2307–2315 (2022). https://doi.org/10.1007/s00520-021-06658-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06658-z