Abstract
Background
Nausea and vomiting are a common clinical symptom in the advanced cancer patient. Pharmacologic management is important. Evidence for drug choices and guidelines are needed to help clinicians manage nausea and vomiting in this population
Methods
Evidence from a systematic review published in 2010, initial MASCC guidelines developed from a systematic review of literature to 2015, and a new systematic review of randomized trials published between 2015 and February 2, 2021, was combined to establish a new guideline.
Results
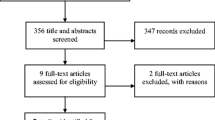
A search of the literature between 2015 and February 2, 2021, revealed 257 abstracts of which there was one systematic review and 4 randomized trials which were used to modify the guideline. The new guideline is as follows: First Line: Metoclopramide (II) multiple small RCTs including a placebo-controlled trial, haloperidol (II) multiple non-placebo-controlled RCTs, high consensus. Second line: Methotrimeprazine (II) 1 well-powered non-placebo-controlled RCT, olanzapine (II) 1 placebo-controlled pilot RCT, high consensus. Third line: Tropisetron (II) large unblinded lower quality non-placebo-controlled RCT, levosulpiride (II) 1 blinded non-placebo-controlled pilot RCT, high consensus.
Discussion
Haloperidol, metoclopramide, methotrimeprazine, olanzapine tropisetron, and levosulpiride have been antiemetics used in randomized trials with antiemetic activity demonstrated. There are only three placebo-controlled randomized trials we could find in our literature review. Placebo responses varied significantly between two randomized trials. More randomized placebo-controlled trials with either metoclopramide or haloperidol rescue are needed to clarify antiemetic choices in advanced cancer.
Conclusion
First-line antiemetics for nausea and vomiting in advanced cancer are metoclopramide and haloperidol, and second-line medications are methotrimeprazine and olanzapine.
Similar content being viewed by others
Data Availability
NA
Code availability
NA
References
Navari RM (2020) Nausea and vomiting in advanced cancer. Curr Treat Options in Oncol 21(2):14
Davis MP, Hallerberg G (2010) Palliative Medicine Study Group of the Multinational Association of Supportive Care in C. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy or radiation. J Pain Symptom Manag 39(4):756–767
Walsh D, Davis M, Ripamonti C, Bruera E, Davies A, Molassiotis A (2017) 2016 Updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer 25(1):333–340
Bruera E, Moyano JR, Sala R, Rico MA, Bosnjak S, Bertolino M, Willey J, Strasser F, Palmer JL (2004) Dexamethasone in addition to metoclopramide for chronic nausea in patients with advanced cancer: a randomized controlled trial. J Pain Symptom Manag 28(4):381–388
Bruera E, Belzile M, Neumann C, Harsanyi Z, Babul N, Darke A (2000) A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manag 19(6):427–435
Bruera ED, MacEachern TJ, Spachynski KA et al (1994) Comparison of the efficacy, safety, and pharmacokinetics of controlled release and immediate release metoclopramide for the management of chronic nausea in patients with advanced cancer. Cancer. 74(12):3204–3211
Corli O, Cozzolino A, Battaiotto L (1995) Effectiveness of levosulpiride versus metoclopramide for nausea and vomiting in advanced cancer patients: a double-blind, randomized, crossover study. J Pain Symptom Manag 10(7):521–526
Mystakidou K, Befon S, Liossi C, Vlachos L (1998) Comparison of the efficacy and safety of tropisetron, metoclopramide, and chlorpromazine in the treatment of emesis associated with far advanced cancer. Cancer. 83(6):1214–1223
Mystakidou K, Befon S, Liossi C, Vlachos L (1998) Comparison of tropisetron and chlorpromazine combinations in the control of nausea and vomiting of patients with advanced cancer. J Pain Symptom Manag 15(3):176–184
Mystakidou K, Befon S, Trifyllis J, Liossi C, Papadimitriou J (1997) Tropisetron versus metoclopramide in the control of emesis in far-advanced cancer. Oncologist. 2(5):319–323
Critchley P, Plach N, Grantham M, Marshall D, Taniguchi A, Bms, Latimer E, Jadad AR (2001) Efficacy of haloperidol in the treatment of nausea and vomiting in the palliative patient: a systematic review. J Pain Symptom Manag 22(2):631–634
Kennett A, Hardy J, Shah S, A’Hern R (2005) An open study of methotrimeprazine in the management of nausea and vomiting in patients with advanced cancer. Support Care Cancer 13(9):715–721
Amesbury B, Alloway L, Hickmore E, Dewhurst G (2004) High-dose levomepromazine (methotrimeprazine) to control nausea in carcinoid syndrome. J Palliat Care 20(2):117–118
Eisenchlas JH, Garrigue N, Junin M, De Simone GG (2005) Low-dose levomepromazine in refractory emesis in advanced cancer patients: an open-label study. Palliat Med 19(1):71–75
Passik SD, Lundberg J, Kirsh KL, Theobald D, Donaghy K, Holtsclaw E, Cooper M, Dugan W (2002) A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manag 23(6):526–532
Skinner J, Skinner A (1999) Levomepromazine for nausea and vomiting in advanced cancer. Hosp Med 60(8):568–570
Homburger F, Smithy G (1954) Chlorpromazine in patients with nausea and vomiting due to advanced cancer. N Engl J Med 251(20):820–822
Fink S, Winslow WA (1955) Anti-emetic effect of chlorpromazine (thorazine) in cancer patients. Gastroenterology. 28(5):731–735
Homburger F, Smithy G (1957) Proclorperazine for the treatment of nausea and vomiting in patients with advanced cancer and other chronic diseases. N Engl J Med 256(1):27
Fletcher DS, Coyne PJ, Dodson PW, Parker GG, Wan W, Smith TJ (2014) A randomized trial of the effectiveness of topical “ABH Gel” (Ativan((R)), Benadryl((R)), Haldol((R))) vs. placebo in cancer patients with nausea. J Pain Symptom Manag 48(5):797–803
Hardy JR, Skerman H, Philip J, Good P, Currow DC, Mitchell G, Yates P (2019) Methotrimeprazine versus haloperidol in palliative care patients with cancer-related nausea: a randomised, double-blind controlled trial. BMJ Open 9(9):e029942
Hardy JR, O’Shea A, White C, Gilshenan K, Welch L, Douglas C (2010) The efficacy of haloperidol in the management of nausea and vomiting in patients with cancer. J Pain Symptom Manag 40(1):111–116
Navari RM, Pywell CM, Le-Rademacher JG et al (2020) Olanzapine for the treatment of advanced cancer-related chronic nausea and/or vomiting: a randomized pilot trial. JAMA Oncol 6(6):895–899
Hui D, Puac V, Shelal Z, Liu D, Maddi R, Kaseb A, Javle M, Overman M, Yennurajalingam S, Gallagher C, Bruera E (2021) Fixed-dose netupitant and palonosetron for chronic nausea in cancer patients: a double-blind, placebo run-in pilot randomized clinical trial. J Pain Symptom Manag 62:223–232.e1
Schwartzberg L, Karthaus M, Rossi G, Rizzi G, Borroni ME, Rugo HS, Jordan K, Hansen V (2019) Fixed combination of oral NEPA (netupitant-palonosetron) for the prevention of acute and delayed chemotherapy-induced nausea and vomiting in patients receiving multiple cycles of chemotherapy: efficacy data from 2 randomized, double-blind phase III studies. Cancer Med 8(5):2064–2073
Aapro M, Jordan K, Gralla RJ, Rizzi G, Rossi G, Palmas M, Alyasova AV, Lisyanskaya AS, Bošnjak SM, Hesketh PJ (2017) Safety and efficacy of NEPA, an oral fixed combination of netupitant and palonosetron, in older patients. J Geriatr Oncol 8(1):56–63
Aapro M, Karthaus M, Schwartzberg L, Bondarenko I, Sarosiek T, Oprean C, Cardona-Huerta S, Hansen V, Rossi G, Rizzi G, Borroni ME, Rugo H (2017) NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy-induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: results of a randomized, double-blind, phase 3 trial versus oral palonosetron. Support Care Cancer 25(4):1127–1135
Baron-Hay S, Aapro M, Bernareggi A, Schwartzberg L (2019) Timing flexibility of oral NEPA, netupitant-palonosetron combination, administration for the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer 27(4):1309–1317
Spinelli T, Calcagnile S, Giuliano C, Rossi G, Lanzarotti C, Mair S, Stevens L, Nisbet I (2014) Netupitant PET imaging and ADME studies in humans. J Clin Pharmacol 54(1):97–108
Sande TA, Laird BJA, Fallon MT (2019) The management of opioid-induced nausea and vomiting in patients with cancer: a systematic review. J Palliat Med 22(1):90–97
Campora E, Merlini L, Pace M, Bruzzone M, Luzzani M, Gottlieb A, Rosso R (1991) The incidence of narcotic-induced emesis. J Pain Symptom Manag 6(7):428–430
Schug SA, Zech D, Grond S (1992) Adverse effects of systemic opioid analgesics. Drug Saf 7(3):200–213
Glare P, Walsh D, Sheehan D (2006) The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care 23(3):229–235
Mallick-Searle T, Fillman M (2017) The pathophysiology, incidence, impact, and treatment of opioid-induced nausea and vomiting. J Am Assoc Nurse Pract 29(11):704–710
Tsukuura H, Miyazaki M, Morita T, Sugishita M, Kato H, Murasaki Y, Gyawali B, Kubo Y, Ando M, Kondo M, Yamada K, Hasegawa Y, Ando Y (2018) Efficacy of prophylactic treatment for oxycodone-induced nausea and vomiting among patients with cancer pain (POINT): a randomized, placebo-controlled, double-blind trial. Oncologist. 23(3):367–374
Hardy J, Daly S, McQuade B, Albertsson M, Chimontsi-Kypriou V, Stathopoulos G, Curtis P (2002) A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 10(3):231–236
Hardy J, Skerman H, Glare P, Philip J, Hudson P, Mitchell G, Martin P, Spruyt O, Currow D, Yates P (2018) A randomized open-label study of guideline-driven antiemetic therapy versus single agent antiemetic therapy in patients with advanced cancer and nausea not related to anticancer treatment. BMC Cancer 18(1):510
Currow DC, Quinn S, Agar M, Fazekas B, Hardy J, McCaffrey N, Eckermann S, Abernethy AP, Clark K (2015) Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manag 49(5):814–821
Feuer DJ, Broadley KE (2000) Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2:CD001219
Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D, Holt T, Morgan S, Lee C, Waite K, Bayman N, Pugh C, Sydes B, Stephens R, Parmar MK, Langley RE (2016) Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 388(10055):2004–2014
Author information
Authors and Affiliations
Contributions
Systematic search: Mellar Davis, David Hui; manuscript development and writing: Mellar Davis, Andrew Davies; editing and critiquing: David Hui, Andrew Davies, Carla Ida Ripamonti, Andreia Capela, Giulia DeFeo, Egidio Del Fabbro, Eduardo Bruera.
Corresponding author
Ethics declarations
Ethics approval
NA
Consent to participate
NA
Consent for publication
NA
Conflict of interest
David Hui grant from Helsinn.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davis, M., Hui, D., Davies, A. et al. MASCC antiemetics in advanced cancer updated guideline. Support Care Cancer 29, 8097–8107 (2021). https://doi.org/10.1007/s00520-021-06437-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06437-w