Abstract
Objectives
This study aimed to investigate the effects of azithromycin suspension on oral mucositis in patients undergoing hematopoietic stem cell transplantation (HSCT).
Methods and material
The study was designed as a single-blind randomized controlled trial in Taleghani medical center affiliated to Shahid Beheshti University of Medical Sciences Tehran Iran. Patients undergoing HSCT were randomly assigned to intervention or control groups. Azithromycin suspension was administered twice daily by gargling for 30 s and swallowing, on the first day of chemotherapy for patients in the intervention group. Graded oral mucositis (OM) occurrence based on National Cancer Institute Common Toxicity Criteria (NCI-CTC) scale (grade 0 to 5) was considered the main outcome, and the Numerical Rating Scale (NRS:0–10) measured the severity of OM symptoms.
Results
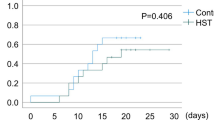
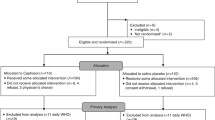
In a duration of 15 months, 88 patients were randomly assigned and finally 70 patients were evaluable for study outcomes (randomized 1:1 to azithromycin versus no-azithromycin). The incidence and duration of the mucositis significantly improved in the intervention group compared to the control. Azithromycin use was consistent with a lower rate of dryness (P < 0.001), dysphagia (P < 0.001), and loss of sense of taste (P < 0.001). Also, in the intervention group, lower intensity of pain due to mucositis (P = 0.01) and lower duration of mucositis were observed (p = 0.045). No significant adverse drug reaction was observed in patients receiving azithromycin.
Conclusion
Based on the result from this study, azithromycin suspension is an effective option in the prevention and treatment of chemotherapy-induced OM. Further study is needed to assess the effect of azithromycin and comparison with other therapeutic options.
Trial registration
Iranian Registry of Clinical Trials: IRCT201603093210N13

Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author (MTA).
Code availability
N/A
References
Barriga F, Ramírez P, Wietstruck A, Rojas N (2012) Hematopoietic stem cell transplantation: clinical use and perspectives. Biol Res 45(3):307–316. https://doi.org/10.4067/S0716-97602012000300012
Hölig K, Kramer M, Kroschinsky F, Bornhäuser M, Mengling T, Schmidt AH et al (2009) Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood 114(18):3757–3763. https://doi.org/10.1182/blood-2009-04-218651
Suárez-Álvarez B, López-Vázquez A, López-Larrea C (2012) Mobilization and homing of hematopoietic stem cells. Adv Exp Med Biol 741:152–170. https://doi.org/10.1007/978-1-4614-2098-9_11
Chen S-H, Wang T-F, Yang K-L (2013) Hematopoietic stem cell donation. Int J Hematol 97(4):446–455. https://doi.org/10.1007/s12185-013-1298-8
Hosseinjani H, Hadjibabaie M, Gholami K, Javadi M, Radfar M, Jahangard-Rafsanjani Z et al (2017) The efficacy of erythropoietin mouthwash in prevention of oral mucositis in patients undergoing autologous hematopoietic SCT: a double-blind, randomized, placebo-controlled trial. Hematol Oncol 35(1):106–112. https://doi.org/10.1002/hon.2250
Pulito C, Cristaudo A, Porta C, Zapperi S, Blandino G, Morrone A et al (2020) Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 39(1):210. https://doi.org/10.1186/s13046-020-01715-7
Lalla RV, Sonis ST, Peterson DE (2008) Management of oral mucositis in patients who have cancer. Dent Clin North Am 52(1):61–77. https://doi.org/10.1016/j.cden.2007.10.002
Mercadante S, Aielli F, Adile C, Ferrera P, Valle A, Fusco F et al (2015) Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Support Care Cancer 23(11):3249–3255. https://doi.org/10.1007/s00520-015-2720-y
Vagliano L, Feraut C, Gobetto G, Trunfio A, Errico A, Campani V et al (2011) Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT—results of a multicentre study. Bone Marrow Transplant 46(5):727–732. https://doi.org/10.1038/bmt.2010.184
Köstler WJ, Hejna M, Wenzel C, Zielinski CC (2001) Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin 51(5):290–315. https://doi.org/10.3322/canjclin.51.5.290
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4(4):277–284. https://doi.org/10.1038/nrc1318
Vanhoecke B, De Ryck T, Stringer A, Van de Wiele T, Keefe D (2015) Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis 21(1):17–30. https://doi.org/10.1111/odi.12224
Zimmermann P, Ziesenitz VC, Curtis N, Ritz N (2018) The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms. Front Immunol 9:302. https://doi.org/10.3389/fimmu.2018.00302
Nozoe K, Aida Y, Fukuda T, Sanui T, Nishimura F (2016) Mechanisms of the macrolide-induced inhibition of superoxide generation by neutrophils. Inflammation 39(3):1039–1048. https://doi.org/10.1007/s10753-016-0333-3
Williams JD (1991) Spectrum of activity of azithromycin. Eur J Clin Microbiol Infect Dis 10(10):813–820. https://doi.org/10.1007/BF01975833
Saad A, de Lima M, Anand S, Bhatt VR, Bookout R, Chen G et al (2020) Hematopoietic cell transplantation, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 18(5):599–634. https://doi.org/10.6004/jnccn.2020.0021
Baden LR, Swaminathan S, Angarone M, Blouin G, Camins BC, Casper C et al (2016) Prevention and Treatment of Cancer-Related Infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 14(7):882–913
Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC et al (2012) Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 67(5):1025–39. https://doi.org/10.6004/jnccn.2016.0093
Shankar A, Roy S, Bhandari M, Rath G, Biswas AS, Kanodia R et al (2017) Current trends in management of oral mucositis in cancer treatment. Asian Pac J Cancer Prev 18(8):2019–2026. https://doi.org/10.22034/APJCP.2017.18.8.2019
Yuen KY, Woo P, Tai J, Lie A, Luk J, Liang R (2001) Effects of clarithromycin on oral mucositis in bone marrow transplant recipients. Haematologica 86(5):554–5. https://doi.org/10.3324/%25x
Ardakani MT, Ghassemi S, Mehdizadeh M, Mojab F, Salamzadeh J, Ghassemi S et al (2016) Evaluating the effect of Matricaria recutita and Mentha piperita herbal mouthwash on management of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized, double blind, placebo controlled clinical trial. Complement Ther Med 29:29–34. https://doi.org/10.1016/j.ctim.2016.08.001
Jahangard-Rafsanjani Z, Gholami K, Hadjibabaie M, Shamshiri A, Alimoghadam K, Sarayani A et al (2013) The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: a randomized clinical trial. Bone Marrow Transplant 48(6):832–836. https://doi.org/10.1038/bmt.2012.250
Funding
The study was conducted under the supervision of deputy of research and technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran, and did not receive any extra source of financial support from secondary institution or company.
Author information
Authors and Affiliations
Contributions
SP, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision; MZ, acquisition of data, drafting of the manuscript, technical support; OM, drafting of the manuscript, statistical analysis, critical revision of the manuscript for important intellectual content; AH, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision; MM, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision; MTA, study concept and design, acquisition of data, drafting of the manuscript, statistical analysis, administrative and material support, critical revision of the manuscript for important intellectual content, study supervision.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the board of ethics committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.PHNM.1394.307).
Consent to participate
The written informed consent was obtained from all patients prior to enrollment.
Consent for publication
The consent for publication of the result of the study was obtained from all of the participants. No personal/contact information of the participant was reported in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parkhideh, S., Zeraatkar, M., Moradi, O. et al. Azithromycin oral suspension in prevention and management of oral mucositis in patients undergoing hematopoietic stem cell transplantation: a randomized controlled trial . Support Care Cancer 30, 251–257 (2022). https://doi.org/10.1007/s00520-021-06409-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06409-0