Abstract
Purpose
Taxane-associated pain syndrome (TAPS) is common with docetaxel and is characterised by myalgias and arthralgias starting 2–3 days after treatment and can last for up to 7 days. Anecdotal evidence suggests that corticosteroids can reduce TAPS. This multicentre, randomized trial evaluated the effect of additional tapering dexamethasone on TAPS.
Methods
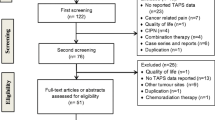
130 breast cancer patients commencing docetaxel were randomized to dexamethasone premedication (8 mg/twice daily for 3 days) or dexamethasone premedication followed by tapering dexamethasone (4 mg/daily for 2 days followed by 2 mg/daily for 2 days). The primary endpoint was absolute change in FACT-Taxane questionnaire during the first chemotherapy cycle. Secondary endpoints: proportion of patients with clinically significant TAPS, QoL, pain and toxicity.
Results
110/130 patients had complete data included in the primary analysis. The fall in FACT-Taxane scores was lower in the experimental group on day 5 (p = 0.05), but not on day 7 (p = 0.21). There was no difference in FACT-Taxane scores over the entire study duration (p = 0.59). Fewer patients in the experimental arm reported TAPS on day 5 (30 vs. 47%). There was a borderline significant attenuation of impairment of QoL with experimental treatment on day 5 (p = 0.06), but not day 7 (p = 0.53). Tapered schedule was associated with more dyspepsia and insomnia.
Conclusion
A tapering schedule of dexamethasone was associated with a brief reduction in docetaxel-associated symptoms which was observed only during dexamethasone exposure and did not persist after discontinuation of the drug.
Trial Registration
ClinicalTrials.gov NCT03348696


Similar content being viewed by others
Data availability
De-identified dataset is available upon request and approval by the Ontario Cancer Research Ethics Board.
Code availability
Not applicable
References
Fernandes R, Mazzarello S, Hutton B, Shorr R, Majeed H, Ibrahim MF, Jacobs C, Ong M, Clemons M (2016) Taxane acute pain syndrome (TAPS) in patients receiving taxane-based chemotherapy for breast cancer-a systematic review. Support Care Cancer 24(8):3633–3650. https://doi.org/10.1007/s00520-016-3256-5
Sanofi-Aventis (2017) Taxotere (docetaxel for injection). http://products.sanofi.ca/en/taxotere.pdf. Accessed September 27 2018
Eckhardt S (1998) The effect of docetaxel on malignant tumors. Orv Hetil 139(15):867–872
Cortes JE, Pazdur R (1995) Docetaxel. J Clin Oncol 13(10):2643–2655
Semb KA, Aamdal S, Oian P (1998) Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J Clin Oncol 16(10):3426–3432. https://doi.org/10.1200/jco.1998.16.10.3426
Chouhan JD, Herrington JD (2011) Single premedication dose of dexamethasone 20 mg IV before docetaxel administration. J Oncol Pharm Pract 17(3):155–159. https://doi.org/10.1177/1078155210367950
Piccart MJ, Klijn J, Paridaens R, Nooij M, Mauriac L, Coleman R, Bontenbal M, Awada A, Selleslags J, Van Vreckem A, Van Glabbeke M (1997) Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol 15(9):3149–3155. https://doi.org/10.1200/jco.1997.15.9.3149
Poi MJ, Berger M, Lustberg M, Layman R, Shapiro CL, Ramaswamy B, Mrozek E, Olson E, Wesolowski R (2013) Docetaxel-induced skin toxicities in breast cancer patients subsequent to paclitaxel shortage: a case series and literature review. Support Care Cancer 21(10):2679–2686. https://doi.org/10.1007/s00520-013-1842-3
Fernandes R, Mazzarello S, Majeed H, Smith S, Shorr R, Hutton B, Ibrahim MF, Jacobs C, Ong M, Clemons M (2016) Treatment of taxane acute pain syndrome (TAPS) in cancer patients receiving taxane-based chemotherapy-a systematic review. Support Care Cancer 24(4):1583–1594. https://doi.org/10.1007/s00520-015-2941-0
Saibil S, Fitzgerald B, Freedman OC, Amir E, Napolskikh J, Salvo N, Dranitsaris G, Clemons M (2010) Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol 17(4):42–47
Chiu N, Zhang L, Dent R, Giotis A, van Draanen J, Gallo-Hershberg D, Chiu L, Chow R, Wan BA, Pasetka M, Stinson J, Stacey E, Verma S, Lam H, Chow E, DeAngelis C (2018) A prospective study of docetaxel-associated pain syndrome. Support Care Cancer 26(1):203–211. https://doi.org/10.1007/s00520-017-3836-z
Jacobs C, Hutton B, Mazzarello S, Smith S, Joy A, Amir E, Ibrahim MF, Gregario N, Daigle K, Eggert L, Clemons M (2015) Optimisation of steroid prophylaxis schedules in breast cancer patients receiving docetaxel chemotherapy-a survey of health care providers and patients. Support Care Cancer 23(11):3269–3275. https://doi.org/10.1007/s00520-015-2731-8
Hilton J, Mazzarello S, Fergusson D, Joy A, Robinson A, Arnaout A, Hutton B, Vandermeer L, Clemons M (2016) Novel methodology for comparing standard-of-care interventions in patients with cancer. J Oncol Pract 12(12):–e1024
ClinicalTrials.gov (2017) Comparing tapering low dose dexamethasone to other standard of care therapies for TAPS in breast cancer patients (REaCT-TAPS). https://clinicaltrials.gov/ct2/show/NCT03348696. Accessed September 27 2018
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579
Cella D, Peterman A, Hudgens S, Webster K, Socinski MA (2003) Measuring the side effects of taxane therapy in oncology: the functional assesment of cancer therapy-taxane (FACT-taxane). Cancer 98(4):822–831. https://doi.org/10.1002/cncr.11578
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L et al (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer (Oxford, England: 1990) 30A(9):1326–1336
McMillan SC, Small BJ, Weitzner M, Schonwetter R, Tittle M, Moody L, Haley WE (2006) Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer 106(1):214–222
McMillan SC, Small BJ (2002) Symptom distress and quality of life in patients with cancer newly admitted to hospice home care. Oncol Nurs Forum 10
Kumar SP (2011) Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care 17(2):108–115
Ramos-Goñi JM, Pinto-Prades JL, Oppe M, Cabasés JM, Serrano-Aguilar P, Rivero-Arias O (2017) Valuation and modeling of EQ-5D-5L health states using a hybrid approach. Med Care 55(7):e51–e58
Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF (2006) Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 94(7):1011–1015. https://doi.org/10.1038/sj.bjc.6603048
Fernandes R, Mazzarello S, Joy AA, Pond GR, Hilton J, Ibrahim MFK, Canil C, Ong M, Stober C, Vandermeer L, Hutton B, da Costa M, Damaraju S, Clemons M (2018) Taxane acute pain syndrome (TAPS) in patients receiving chemotherapy for breast or prostate cancer: a prospective multi-center study. Support Care Cancer 26(9):3073–3081. https://doi.org/10.1007/s00520-018-4161-x
Fernandes R, Mazzarello S, Hutton B, Shorr R, Ibrahim MF, Jacobs C, Ong M, Clemons M (2017) A systematic review of the incidence and risk factors for taxane acute pain syndrome in patients receiving taxane-based chemotherapy for prostate cancer. Clin Genitourin Cancer 15(1):1–6
Clemons M, Mazzarello S, Hilton J, Joy A, Price-Hiller J, Zhu X, Verma S, Kehoe A, Ibrahim MF, Sienkiewicz M, Stober C, Vandermeer L, Hutton B, Mallick R, Fergusson D (2019) Feasibility of using a pragmatic trials model to compare two primary febrile neutropenia prophylaxis regimens (ciprofloxacin versus G-CSF) in patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer (REaCT-TC). Support Care Cancer 27(4):1345–1354. https://doi.org/10.1007/s00520-018-4408-6
Ibrahim MFK, Hilton J, Mazzarello S, Fergusson D, Hutton B, Robinson A, Califaretti N, Hsu T, Gertler S, Mates M, Stober C, Vandermeer L, Mallick R, Clemons M (2018) A multi-center pragmatic, randomized, feasibility trial comparing standard of care schedules of filgrastim administration for primary febrile neutropenia prophylaxis in early-stage breast cancer. Breast Cancer Res Treat 168(2):371–379. https://doi.org/10.1007/s10549-017-4604-y
Kim SY, Miller FG (2014) Informed consent for pragmatic trials—the integrated consent model. N Engl J Med 370(8):769–772. https://doi.org/10.1056/NEJMhle1312508
Kim SY, Miller FG (2015) Varieties of standard-of-care treatment randomized trials: ethical implications. JAMA 313(9):895–896. https://doi.org/10.1001/jama.2014.18528
Health"; OMo (2017) Schedule of Benefits for Physician Services Under the Health Insurance Act 2015
Acknowledgements
We are grateful to the patients, their families and their physicians for their participation in this study. Accrual by physician was: Clemons (84), Simos (23), Ng (6), Zibdawi (6), Hsu (3), Srikanthan (2), Gertler (2), Sehdev (1) Hilton (1) Rana (2).
Funding
Funding of this study was through the Rethinking Clinical Trials (REaCT program) and the Cancer Care Ontario - Clinical Programs and Quality Initiatives grant (Government of Ontario, 2017) to support the expansion of REaCT trials to other cancer centres in Ontario.
Author information
Authors and Affiliations
Consortia
Contributions
MC, DF, LV, BH, and EA designed the study and prepared the protocol. MC, DSimos, MS, TN, LZ, BB, AA, and DSaunders collected the data. MC acted as principal investigator, MS and DSaunders coordinated data entry and EA did the statistical analysis. MC, MS, LV, DSaunders and EA had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MC, MS, DSaunders, and EA wrote the manuscript. All authors were involved in the critical review of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and the Ontario Cancer Research Ethics Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Integrated informed consent was obtained from all trial participants included in the study.
Consent for publication
The participants gave consent to de-identified publication of aggregate study results.
Conflict of interest
MC reports personal fees (honoraria) from Pfizer, outside the submitted work. TN reports personal fees (honoraria) from ARIAD, Takeda and Boehringer-Ingelheim, outside the submitted work. AA has participated on an advisory board for Novartis, Eli Lily, Exactis innovation and Pfizer, has received honoraria from Apobiologix and Roche and has received travel funds from Roche. BH reports consulting fees from Cornerstone Research, outside the submitted work. All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Clemons, M., Simos, D., Sienkiewicz, M. et al. A prospective multi-centre, randomized study comparing the addition of tapering dexamethasone to other standard of care therapies for taxane-associated pain syndrome (TAPS) in breast cancer patients. Support Care Cancer 29, 5787–5795 (2021). https://doi.org/10.1007/s00520-021-06142-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06142-8