Abstract
Background
The large number of women surviving many years post breast cancer (BC) diagnosis has heightened interest in studying long-term effects of cancer on quality of life (QoL). Several cancer-specific health-related measures have been developed, but these may not be appropriate for long survivors. This study evaluates the reliability and clinical and psychometric validity of the BreSAS questionnaire (BQ) among BC survivors.
Methods
The BQ is a quick, simple ten-item module for the assessment of long-term physical, psychological, sexual, and cognitive effects that may influence QoL. The total BreSAS score ranks from 0 to 100, with a low score indicating a better QoL. Patients complete the BQ, the FACT-ES questionnaire, and case report forms for clinical and socio-demographic data during follow-up visits. Reliability and clinical and psychometric validity of the questionnaires are assessed by correlation analyses and exploration of known group comparisons.
Results
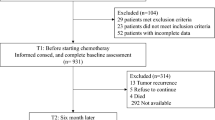
From September 2015 to February 2016, 149 patients from three Italian oncology units were enrolled. Baseline questionnaires were returned from all, and 134 patients (89%) completed the BQ and FACT-ES in less than 15 min. For reliability, Cronbach’s alpha coefficients for each scale were greater than 0.70 in all analyzed symptoms. Convergent validity of BQ showed by Pearson’s r demonstrated a high correlation between intensity of symptoms and QoL, especially for pain and depression. No data were provided about reproducibility with test–retest study.
Conclusion
The BQ demonstrates sufficient validity and reliability to support its use to assess patient-reported symptoms during planned follow-up clinical visits among BC survivors. Further full validation studies are needed.
Similar content being viewed by others
References
Carioli G, Malvezzi M, Rodriguez T, Bertuccio P, Negri E, la Vecchia C (2017) Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 36:89–95
American Cancer Society (2005) Cancer facts and figures 2005. American Cancer Society, Atlanta
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivor. Accessed 10 April 2019
Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE (1998) Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol 16(2):501–514
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991 Summer) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7(2):6–9
Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55(2):189–199
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997 Mar) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15(3):974–986
Fayers PM, Machin D (2000) Quality of life: assessment, analysis and interpretation. John Wiley & Sons Ltd., Chichester 404 pp
Tabachnik BJ, Fidel LS (1993) Using multivariate statistics. Harper & Row, London
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The EORTC QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Schipper H, Clinch J, McMurray A, Levitt M (1984) Measuring the quality of life of cancer patients: the Functional Living Index-Cancer: development and validation. J Clin Oncol 2:472–483
Schag CA, Heinrich RL (1990) Development of a comprehensive quality of life measurement tool: CARES. Oncology (Williston Park) 4:135–138
Wyatt G, Friedman LL (1996) Long-term female cancer survivors: quality of life issues and clinical implications. Cancer Nurs 19:1–7
Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P (1995) Quality of life in long-term cancer survivors. Oncol Nurs Forum 22:915–922
Avis NE, Ip E, Foley KL (2006) Evaluation of the Quality of Life in Adult Cancer Survivors (QLACS) scale for long-term cancer survivors in a sample of breast cancer survivors. Health Qual Life Outcomes 4(1):92
Brandini da Silva FC, José da Silva J, Sarri AJ, Paiva CE, Aloisio da Costa Vieira R (2019) Comprehensive validation study of quality-of-life questionnaire using objective clinical measures: breast Cancer treatment outcome scale (BCTOS), Brazilian Portuguese version. Clin Breast Cancer 19(1):e85–e100
Acknowledgments
We want to thank all the participants of the study for the additional time required to complete all the study questionnaires during the follow-up visit.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giusti, R., Scarpi, E., Cannita, K. et al. Clinical and psychometric validation of the BreSAS questionnaire module for symptom assessment among breast cancer survivors. Support Care Cancer 28, 1051–1058 (2020). https://doi.org/10.1007/s00520-019-04905-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04905-y