Abstract
Purpose
To present the findings of combined oral assessment and gustometry testing of a series of head and neck and hematologic malignancies in patients with self-reported taste change after cytotoxic therapies.
Methods
Patients with acute myeloid leukemia (AML), multiple myeloma (MM), and head and neck cancer (HNC) were evaluated for taste function. Chemical gustometry was conducted assessing chemosensory qualities that included sweet, sour, salty, bitter, umami, and spicy. NCI Common Terminology Criteria for Adverse Events (CTCAE) 4.0 and the Scale of Subjective Total Taste Acuity (STTA) were used to describe taste symptoms. Saliva flow rates were measured to determine the presence of hyposalivation. Patients were provided treatment trials for taste dysfunction, including zinc supplements, or medications that included clonazepam, megestrol acetate, and the cannabinoid dronabinol.
Results
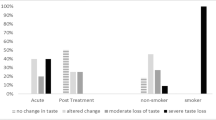
According to STTA, hematology cases reported the incidence of grades 2 and 3 taste disturbances as 60% and 40%, respectively. For HNC patients, the incidence of grades 2 and 3 was 44% each. Gustometry tests confirmed dysgeusia in all patients evaluated. In the hematology group, 80% of patients exhibited a decrease in sweet taste perception, and no patients correctly identified umami taste. In the HNC group, most patients could not identify salt taste, 66% of patients reported “no sensation” with spicy taste, bitter taste was reduced in some, and increased or altered in others, while only one patient could identify umami taste. In the hematologic and HNC patient groups, 80% and 66% reported grade 2 dry mouth, respectively, according to CTCAE 4.0. After treatment for taste dysfunction, 71% of all patients in the present study reported improvements in taste function.
Conclusions
Persisting dysgeusia in cancer survivors may be assessed by patient report and taste testing. The taste most affected in our patients was umami. Treatment trials with current interventions for dysgeusia appeared effective and should be considered in cancer survivors. Understanding taste and flavor function during and following cancer treatment is important in developing rational prospective preventive and interventional strategies.
Similar content being viewed by others
References
Epstein JB, Smutzer G, Doty RL (2016) Understanding the impact of taste changes in oncology care. Support Care Cancer 24:1917–1931
Nguyen HM, Reyland ME, Barlow LA (2012) Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci 32:3474–3484
Hovan AJ, Williams PM, Stevenson-Moore P et al (2010) A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer 18:1081–1087
Epstein JB, Barasch A (2010) Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol 46:77–81
Riedel K, Sombroek D, Fiedler B, Siems K, Krohn M (2017) Human cell-based taste perception—a bittersweet job for industry. Nat Prod Rep 34:484–495
Hawkes CH (2002) Anatomy and physiology of taste sense. Smell and taste complaints. Butterworth Heinemann, Amsterdam, pp 123–45
Bromley SM, Doty RL (2015) Clinical disorders affecting taste: an update. In: Doty RL (ed) Handbook of olfaction and gustation, 3rd edn. John Wiley & Sons, Hoboken, pp 887–910
Boyce JM, Shone GR (2006) Effects of ageing on smell and taste. Postgrad Med J 82:239–241
Abasaeed R, Coldwell SE, Lloid ME, Soliman SH, Macris PC, Schubert MM (2018) Chemosensory changes and quality of life in patients undergoing hematopoietic stem cell transplantation. Support Care Cancer 26:3552–3561
Boltong A, Keast R, Aranda S (2012) Experiences and consequences of altered taste, flavour and food hedonics during chemotherapy treatment. Support Care Cancer 20:2765–2774
Jensen SB, Mouridsen HT, Bergmann OJ, Reibel J, Brunner N, Nauntofte B (2008) Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:217–226
IJpma I, Renken RJ, Ter Horst GJ, Reyners AK (2015) Metallic taste in cancer patient treated with chemotherapy. Cancer Treat Rev 41:179–186
Schiffman SS, Warwick ZS (1993) Effect of flavor enhancement of foods for the elderly on nutritional status: food intake, biochemical indices, and anthropometric measures. Physiol Behav 53:395–402
Doty RL, Bromley SM (2004) Effects of drugs on olfaction and taste. Otolaryngol Clin N Am 37:1229–1254
Smutzer G, Lam S, Hastings L, Desai H, Abarintos RA, Sobel M, Sayed N (2008) A test for measuring gustatory function. Laryngoscope 118:1411–1416
Smutzer G, Jacob JC, Tran JT, Shah DI, Gambhir S, Devassy RK, Tran EB, Hoang BT, McCune JF (2018) Detection and modulation of capsaicin perception in the human oral cavity. Physiol Behav 194:120–131
Epstein JB, Beier Jensen S (2015) Management of hyposalivation and xerostomia: criteria for treatment strategies. Compend Contin Educ Dent 36:600–603
Doty RL, Chen JH, Overend J (2017) Taste quality confusions: influences of age, smoking, PTC taster status, and other subject characteristics. Perception 46:257–267
Scordo M, Shah GL, Peled JU, Preston EV, Buchan ML, Epstein JB, Barasch A, Giralt SA (2018) Unlocking the complex flavors of dysgeusia after hematopoietic cell transplantation. Biol Blood Marrow Transplant 24:425–432
Hull KM, Kerridge I, Schifter M (2012) Long-term oral complications of allogeneic haematopoietic SCT. Bone Marrow Transplant 47:265–270
Iestra JA, Fibbe WE, Zwinderman AH, Van Staveren WA, Kromhout D (2002) Body weight recovery, eating difficulties and compliance with dietary advice in the first year after stem cell transplantation: a prospective study. Bone Marrow Transplant 29:417–424
Boer CC, Correa ME, Miranda EC, de Souza CA (2010) Taste disorders and oral evaluation in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant 45:705–711
Mattsson T, Arvidson K, Heimdahl A, Ljungman P, Dahllof G, Ringden O (1992) Alterations in taste acuity associated with allogeneic bone marrow transplantation. J Oral Pathol Med 21:33–37
Epstein JB, Phillips N, Parry J, Epstein MS, Nevill T, Stevenson-Moore P (2002) Quality of life, taste, olfactory and oral function following high-dose chemotherapy and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 30:785–792
Marinone MG, Rizzoni D, Ferremi P, Rossi G, Izzi T, Brusotti C (1991) Late taste disorders in bone marrow transplantation: clinical evaluation with taste solutions in autologous and allogeneic bone marrow recipients. Haematologica 76:519–522
Ruo Redda MG, Allis S (2006) Radiotherapy induced taste impairment. Cancer Treat Rev 32:541–547
Jensen SB, Pedersen AM, Vissink A et al (2010) A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer 18:1039–1060
Oates JE, Clark JR, Read J, Reeves N, Gao K, Jackson M, Boyer M, O’Brien CJ (2007) Prospective evaluation of quality of life and nutrition before and after treatment for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 133:533–540
Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, Stevenson-Moore P, Osoba D (1999) Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck 21:1–11
Yamashita H, Nakagawa K, Tago M, Nakamura N, Shiraishi K, Eda M, Nakata H, Nagamatsu N, Yokoyama R, Onimura M, Ohtomo K (2006) Taste dysfunction in patients receiving radiotherapy. Head Neck 28:508–516
Comeau TB, Epstein JB, Migas C (2001) Taste and smell dysfunction in patients receiving chemotherapy: a review of current knowledge. J Support Care Cancer 9:575–580
Yamashita H, Nakagawa K, Hosoi Y, Kurokawa A, Fukuda Y, Matsumoto I, Misaka T, Abe K (2009) Umami taste dysfunction in patients receiving radiotherapy for head and neck cancer. Oral Oncol 45:e19–e23
Shi HB, Masuda M, Umezaki T, Kuratomi Y, Kumamoto Y, Yamamoto T, Komiyama S (2004) Irradiation impairment of umami taste in patients with head and neck cancer. Auris Nasus Larynx 31:401–406
Yamashita H, Nakagawa K, Nakamura N, Abe K, Asakage T, Ohmoto M, Okada S, Matsumoto I, Hosoi Y, Sasano N, Yamakawa S, Ohtomo K (2006) Relation between acute and late irradiation impairment of four basic tastes and irradiated tongue volume in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 66:1422–1429
Thorne T, Olson K, Wismer W (2015) A state-of-the-art review of the management and treatment of taste and smell alterations in adult oncology patients. Support Care Cancer 23:2843–2851
Heckmann SM, Hujoel P, Habiger S, Friess W, Wichmann M, Heckmann JG, Hummel T (2005) Zinc gluconate in the treatment of dysgeusia—a randomized clinical trial. J Dent Res 84:35–38
Takaoka T, Sarukura N, Ueda C, Kitamura Y, Kalubi B, Toda N, Abe K, Yamamoto S, Takeda N (2010) Effects of zinc supplementation on serum zinc concentration and ratio of apo/holo-activities of angiotensin converting enzyme in patients with taste impairment. Auris Nasus Larynx 37:190–194
Lyckholm L, Heddinger SP, Parker G, Coyne PJ, Ramakrishnan V, Smith TJ, Henkin RI (2012) A randomized, placebo controlled trial of oral zinc for chemotherapy-related taste and smell disorders. J Pain Palliat Care Pharmacother 26:111–114
Cui Y, Xu H, Chen FM, Liu JL, Jiang L, Zhou Y, Chen QM (2016) Efficacy evaluation of clonazepam for symptoms remission in burning mouth syndrome: a meta-analysis. Oral Dis 22:503–511
Heckmann SM, Kirchner E, Grushka M, Wichmann MG, Hummel T (2012) A double-blind study on clonazepam in patients with burning mouth syndrome. Laryngoscope 122:813–816
Brisbois TD, de Kock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M, MacDonald N, Baracos VE, Wismer WV (2011) Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol 22:2086–2093
De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G (2012) Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology. 63:161–168
Epstein JB, Raber-Durlacher JE, Lill M, Linhares YP, Chang J, Barasch A, Slief RJ, Geuke M, Zecha JM, Milstein DM, Tzachanis D (2017) Photobiomodulation therapy in the management of chronic graft-versus-host disease. Support Care Cancer 25:357–364
Bjordal JM, Bensadoun RJ, Tuner J, Frigo L, Gjerde K, Lopes-Martins RA (2011) A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support Care Cancer 19:1069–1077
Heintze U, Birkhed D, Bjorn H (1983) Secretion rate and buffer effect of resting and stimulated whole saliva as a function of age and sex. Swed Dent J 7:227–238
Funding
Taste strips were developed with funding from a Targeted Small Grant from Temple University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Epstein, J.B., de Andrade e Silva, S.M., Epstein, G.L. et al. Taste disorders following cancer treatment: report of a case series. Support Care Cancer 27, 4587–4595 (2019). https://doi.org/10.1007/s00520-019-04758-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04758-5



