Abstract
Purpose
Afatinib is a standard first-line therapy for advanced EGFR-positive NSCLC. We implemented a pharmacist-led proactive follow-up algorithm to identify and manage early afatinib-related adverse events (AEs).
Methods
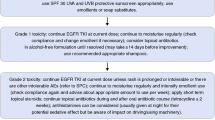
We conducted a retrospective chart review of all patients treated with afatinib after implementation of the algorithm at the Sunnybrook Odette Cancer Centre (Toronto, ON, Canada) from April 1, 2015 to July 31, 2016. Our in-house algorithm involved consultations in person and proactive pharmacist-led callbacks on days 5, 10, and 17. All AEs were graded and documented in real time and management based on toxicity grade was standardized. This study evaluated the impact of our algorithm on real-world AEs.
Results and discussion
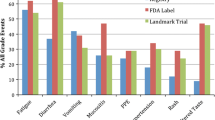
Thirty-three patients were identified and reviewed. Median follow-up was 248 days. All patients experienced at least one drug-related AE; 18.2% were grade 3/4. The most common AEs were diarrhea 87.9%, rash 81.8%, stomatitis 57.6%, and paronychia 45.5%. Median dose of afatinib was 40 mg daily; 51.5% of patients had ≥ 1 dose reduction and 6.3% discontinued afatinib due to AEs. Proactive calls by the pharmacist identified 36.5% of all drug-related AEs, 33.3% of grade 3/4 AEs, 58.1% of first drug-related AEs and identified two patients that were non-compliant. Only 3.2% of AEs were identified by an emergency room/urgent clinic visit.
Conclusions
This proactive multi-disciplinary AE management algorithm resulted in a low rate of urgent assessments and discontinuation due to toxicity while maintaining afatinib at ideal dose, thus providing a useful tool for centers prescribing afatinib.
Similar content being viewed by others
References
Siegel R, Ma J, Zou Z et al (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Carey KD (2006) Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor. Erlotinib Cancer Res 66:8163–8171
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. JNCI J Natl Cancer Inst 97:339–346
Barlesi F, Blons H, Beau-Faller M et al (2013) Biomarkers (BM) France: results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol 31:8000–8000
Zhu CQ, da Cunha SG, Ding K et al (2008) Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 26:4268–4275
Zhang YL, Yuan JQ, Wang KF et al (2016) The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 7:78985–78993
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, Doebele RC, Govindan R, Gubens MA, Hennon M, Horn L, Komaki R, Lackner RP, Lanuti M, Leal TA, Leisch LJ, Lilenbaum R, Lin J, Loo BW Jr, Martins R, Otterson GA, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M (2017) Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 15:504–535
Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller JH, Smith TJ, Strawn JR, Trent D, Johnson DH (2015) Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3488–3515
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncology 11:121–128
Mok TS, Wu Y-L, Thongprasert S et al (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Rosell R, Gervais R, Vergnenegre A, Massuti B, Felip E, Cardenal F, Garcia Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, di Seri M, Garrido Lopez P, Insa A, de Marinis F, Corre R, Carreras M, Carcereny E, Taron M, Paz-Ares LG, Spanish Lung Cancer Group (2011) Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: interim results of the European erlotinib versus chemotherapy (EURTAC) phase III randomized trial. J Clin Oncol 29:7503–7503
Sequist LV, Yang JC, Yamamoto N et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327–3334
Wu Y-L, Zhou C, Hu C-P, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15:213–222
Zhou C, Wu Y-L, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742
Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, Kraemer O, Himmelsbach F, Haaksma E, Adolf GR (2012) Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 343:342–350
Yang JC, Sequist LV, Zhou C et al (2016) Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 27:2103–2110
Paz-Ares L, Tan EH, O'Byrne K et al (2017) Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 28:270–277
Park K, Tan E-H, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JCH, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, Kim DW, Arvis CD, Kölbeck K, Laurie SA, Tsai CM, Shahidi M, Kim M, Massey D, Zazulina V, Paz-Ares L (2016) Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 17:577–589
Passaro A, Di Maio M, Del Signore E, et al: Management of nonhematologic toxicities associated with different EGFR-TKIs in advanced NSCLC: a comparison analysis. Clin Lung Cancer 15:307–312, 2014
Liu G, Franssen E, Fitch MI, Warner E (1997) Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 15:110–115
Schellens JH (2005) Challenges of oral chemotherapy. Clin Adv Hematol Oncol 3:99–100
McLeod HL, Evans WE (1999) Oral cancer chemotherapy: the promise and the pitfalls. Clin Cancer Res 5:2669–2671
Lam MS, Cheung N (2016) Impact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemia. J Oncol Pharm Pract 22:741–748
Kearney N, McCann L, Norrie J, Taylor L, Gray P, McGee-Lennon M, Sage M, Miller M, Maguire R (2009) Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer 17:437–444
Hirsh V (2011) Managing treatment-related adverse events associated with egfr tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol 18:126–138
Melosky B, Leighl NB, Rothenstein J, Sangha R, Stewart D, Papp K (2015) Management of egfr tki–induced dermatologic adverse events. Curr Oncol 22:123–132
Afatinib - CCO formulary - August 2017. Available at: https://cancercare.on.ca/CCO_DrugFormulary/pages/DfPdfContent.aspx?cat=DM&name=afatinib. Accessed August 7, 2017
Afatinib - BC Cancer Agency Cancer Drug Manuale. Available at: http://webcache.googleusercontent.com/search?q=cache:pXC2Fxjuy18J:www.bccancer.bc.ca/drug-database-site/Drug%2520Index/Afatinib_monograph_1Dec2014.pdf+&cd=1&hl=en&ct=clnk&gl=us. Accessed August 7, 2017
Lacouture ME, Anadkat MJ, Bensadoun RJ et al (2011) Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 19:1079–1095
Pan-Canadian Oncology Symptom Triage and Remote Support (COSTaRS) Team.: Remote Symptom Practice Guides for Adults on Cancer Treatments - COSTaRS Symptom Assessment. https://ktcanada.ohri.ca/costars/COSTaRS_Practice_Guides_ENGLISH_March2016.pdf; Accessed July 16, 2017
Cancer Care Ontario: Symptom Management Guides. Available at: https://www.cancercare.on.ca/toolbox/symptools/patient_symptom_management_guides/. Accessed August 30, 2017
Cancer Care Ontario: Algorithm: Diarrhea Symptoms in Adults with Cancer. Available at: https://www.cancercare.on.ca/CCO_DrugFormulary/Pages/FileContent.aspx?fileId=154789. Accessed August 30, 2017
Cancer Care Ontario: Algorithm: Mucositis in Adults with Cancer: Screening and Assessment. Available at: https://www.cancercare.on.ca/CCO_DrugFormulary/Pages/FileContent.aspx?fileId=154817. Accessed August 30, 2017
Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, Xu F, Yassine M (2010) Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 28:1351–1357
Melosky B, Anderson H, Burkes RL, Chu Q, Hao D, Ho V, Ho C, Lam W, Lee CW, Leighl NB, Murray N, Sun S, Winston R, Laskin JJ (2016) Pan Canadian rash trial: a randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor-induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol 34:810–815
Berlin JA, Glasser SC, Ellenberg SS (2008) Adverse event detection in drug development: recommendations and obligations beyond phase 3. Am J Public Health 98:1366–1371
Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ (2011) Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 17:6298–6303
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, Chou JF, Dulko D, Sit L, Barz A, Novotny P, Fruscione M, Sloan JA, Schrag D (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34:557–565
Shah A Maroun J, Dranitsaris G. The cost of hospitalization secondary to severe chemotherapy induced diarrhea (CID) in patients with colorectal cancer. J Clin Oncol. 2016: 22 (14)_suppl pp. 6111–6113
Tai E, Guy G, Dunbar A et al (2017) Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract 13(6):e552–e561
Neuss MN, Gilmore TR, Belderson KM, et al: 2016 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards, including standards for pediatric oncology. Oncol Nurs Forum 44:31–43, 2017
Acknowledgments
We would like to thank Paul Card and Loretta Collins from Kaleidoscope Strategic Inc. for their editorial support in preparation of this paper.
Funding
This study was funded by an unrestricted research grant from Boehringer Ingelheim, Canada.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
Boehringer Ingelheim Canada supported this study with an unrestricted educational grant. Funds were used for data collection, statistical analysis, and writing of our retrospective analysis. Boehringer Ingelheim had no input on the follow-up algorithm, the data results, or interpretation of the analysis. We have full control of the primary data and will allow the journal to review our data if requested. Dr. Cheema, Alia Thawer, and Dr. Suneil Khanna have participated in advisory boards for Boehringer Ingelheim.
Disclaimer
This review was prepared according to ICMJE standards with editorial assistance from Kaleidoscope Strategic Inc.
Rights and permissions
About this article
Cite this article
Cheema, P.K., Thawer, A., Leake, J. et al. Multi-disciplinary proactive follow-up algorithm for patients with advanced NSCLC receiving afatinib. Support Care Cancer 27, 1029–1039 (2019). https://doi.org/10.1007/s00520-018-4392-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4392-x